Week 8 Problem Set 30BL
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
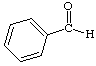
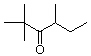
1. How many alpha hydrogens do each of the following compounds have? huh?
| a. |
c.  |
b.  |
d. |
2. Give the expected product for the following base catalyzed aldol condensations with dehydration reactions.
a.
b. 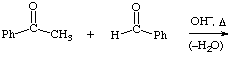 help
help
c. 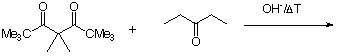 hint
hint
d. 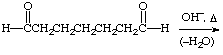
3. Why is absolute ethanol used as solvent for the reaction and not 95% ethanol? Explain.
4. If 400 mg of crude product, 1,2,3,4-tetraphenylcyclopentadienone, is isolated, approximately how much solvent in needed for the recrystallization? How much (in gram and percentage) is recovered after the recystallization? (assume that the recrystallizing solvent is a 1:1 mixture of 95% ethanol and toluene). Given: solubility of product = 25 mg/mL hot solvent and 2 mg/mL cold solvent.
5. The final product, tetraphenylcylcopentadienone is recrystallized from a 1:1 mixture of 95% ethanol and toluene. What would be expected if only 95% ethanol was used for the recystallization? What would be expected if only toluene was used for the recystallization?
6. True or false? Explain briefly.
a. A solvent that has a flat solubility curve is a good solvent for recrystallization.
b. It is enough to warm up the solvent gently to dissolve the compound.
c. It doesn't matter how much solvent is added as long as it dissolves all the compound.
d. The crystals of this week's product can be rinsed with toluene.
e. Black boiling stones can be used to avoid bumping during the recrystallization.
7. TLC
The researcher attempts to evaluate the purity of his product by TLC.
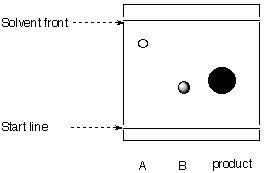 |
stationary phase: silica mobile phase: toluene |
a. Calculate the Rf-values for A, B and C.
b. What can be said about the purity of C?
c. Is the chromatogram obtained correctly?
d. What are the relative polarities of A, B and C?