 .
If you decide to drop the course after the first meeting, you are still
obligated to attend the second meeting to dispose of the reaction mixture. If you do not do this, you will be charge a fee for abandoning your desk.
.
If you decide to drop the course after the first meeting, you are still
obligated to attend the second meeting to dispose of the reaction mixture. If you do not do this, you will be charge a fee for abandoning your desk.Problem Set for Meeting #1
Announcements
1. There is not prelab due in the meeting 1. The prelab for the benzoin experiment will have to be submitted to the TA during meeting 2.
2. THIS PROBLEM SET SHOULD BE COMPLETED DURING YOUR ASSIGNED LAB SECTION. You will not have to turn in anything in the end for the meeting 1 assignment, but you will have have to use the numbers that you generate for meeting 2 homework.
Assignment
1. Learn how to access and use the course website. Note that there are many useful hints posted on the course website that can make your life much easier in this course i.e., Rules 1_50_ for_O-Chem lab (Flash card version), technique videos, etc. You should check prior EACH meeting if there is any relevant information posted on how to conduct the experiment and other tutorials (and videos) about specific techniques. This will help you to get a better handle on the material and make the experiment run smoother.
2. Locate the eye wash/shower (in the lab and hallway), phone, fire extinguishers and emergency exits. In cases of emergency call 911 from a campus phone or (310)825-1491 (from cell phone). Both numbers will connect you to with the campus police.
3. Start the coenzyme synthesis of benzoin.
Make sure to close the drum vial properly, label it and store it in the
drawer. Come back after two days to check if the crystals were formed
(if the lab is closed, see the instructor for the key). If crystals did
not form, the student should scratch the bottom and the lower sides of
the vial with a glass rod on the inside, but be careful not to poke a hole into the
vial
 .
If you decide to drop the course after the first meeting, you are still
obligated to attend the second meeting to dispose of the reaction mixture. If you do not do this, you will be charge a fee for abandoning your desk.
.
If you decide to drop the course after the first meeting, you are still
obligated to attend the second meeting to dispose of the reaction mixture. If you do not do this, you will be charge a fee for abandoning your desk.
4. Use PC Spartan 2014 to draw the structures below.
a. Open only one window at a time in Spartan 2014. Too many windows can cause problems for everybody using the program because the server sometimes thinks that there are many more users. Since the number of licenses in the SLC is limited, some of the students might not be able to start the program. The screen should look like this after you start the program. If the program does not start up, please consult your TA. If s/he cannot fix the problem, Marek Grzeskowiak (YH 4356, last room on the left-hand side) should be consulted because he is the system administrator for the SLC.
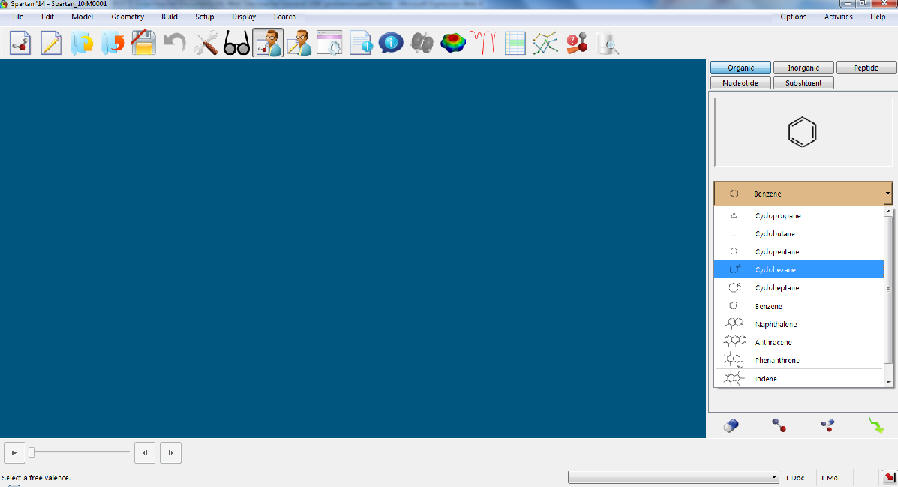
b. If you need (or want) to start over, close the scratch file first (without saving it) before you draw a new molecule.
c. Use the available templates i.e., rings, etc. (see right hand side panel, use the 'Organic' model kit) to speed up the process of drawing a molecule. There is no need to re-invent anything (i.e., cyclohexane rings, benzene rings, etc). It is highly advisable to draw the basic framework first and add the peripheral groups later on. The easiest way is to just click at the end of the open valence.
d. Make sure that you save the files in your own network folder. If you do work in pairs, one of you will have to draw them again later because they are used throughout the quarter in homework assignments and the molecular modeling labs.
e. In order to draw the borneol, isoborneol and camphor, draw methylcyclohexane first and then connect the methyl group with the carbon atom in 4-position to form the bicycle. The initial structure (shown on the left) has to be be minimized before attaching the other groups (minimized structure shown on the right). This will make it easier to see the basic structure. The mimimization feature only normalizes the the bond length and bond angle. Thus, the structure obtained follows some very basic rules and does not represent the true structure of the molecule in terms of bond angles, bond distances, torsion angles, etc. As a the result, the properties predicted based on this structure are not very reliable.
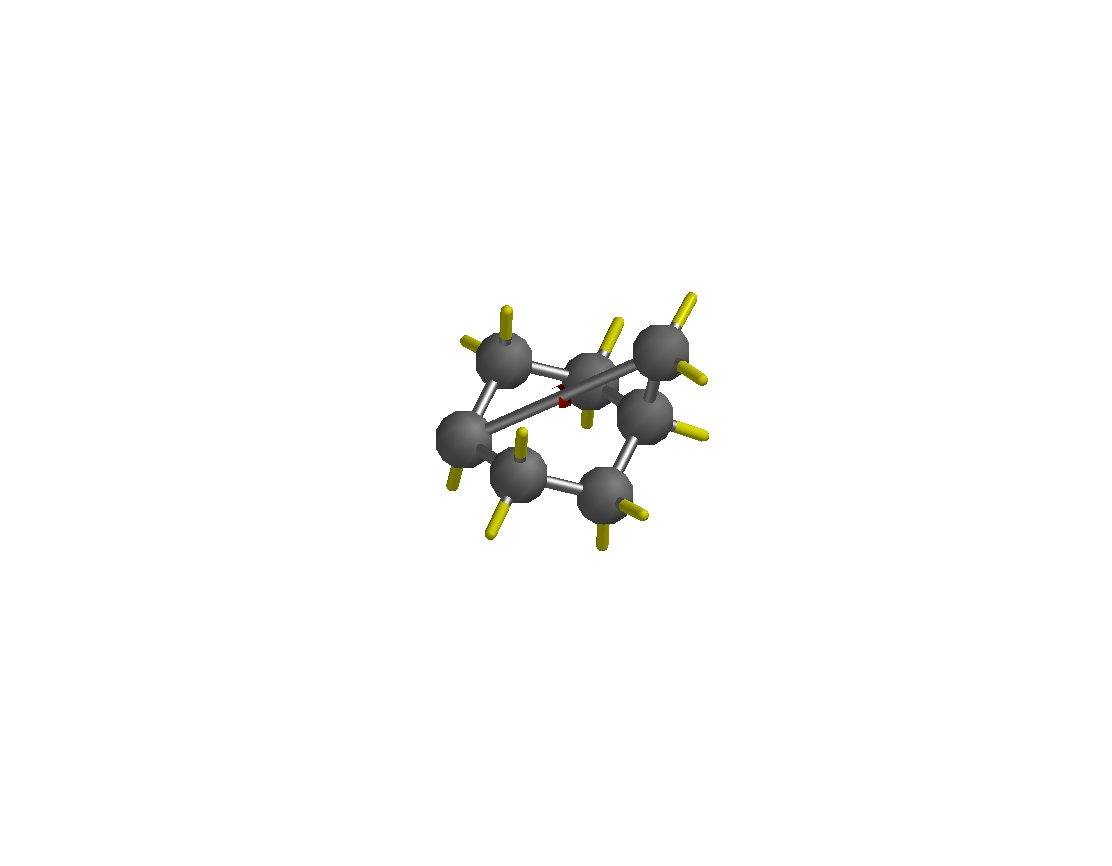
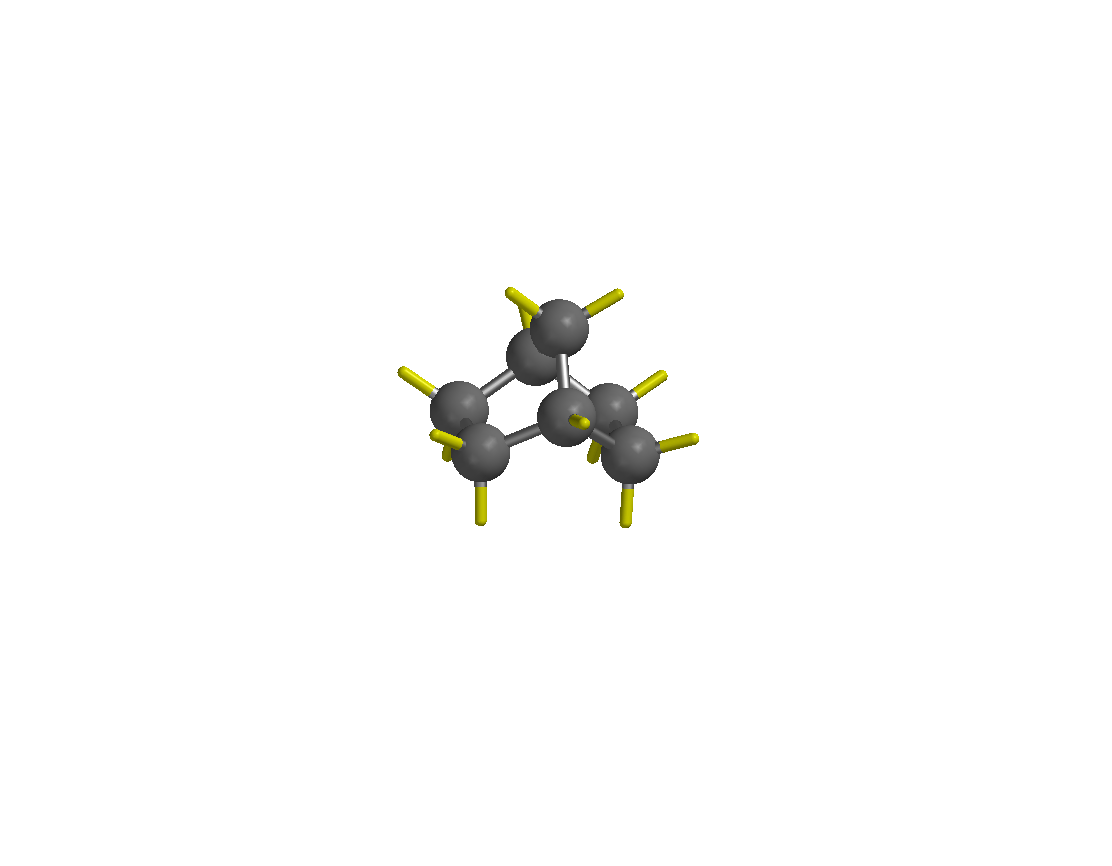
f. You do not have to draw each hydrogen atom separately. All valences that are not defined otherwise are considered hydrogen atoms by default in this program (i.e., the yellow sticks in the diagrams above). Make sure that you do not delete any of them by accident because this will cause problems later when you try to perform calculations since the molecule might be a radical or diradical then. The multiplicity of the molecule will be wrong, which causes any calculations to fail! ![]()
h. It is highly advisable to minimize the structures frequently to get the proper arrangements of the atoms in a larger structure.
i. You will not have to print out the structures, but it is highly advisable to get them checked by the teaching assistant or the instructor before you leave. This will minimize problems later on when you work on your own.
j. In some cases, the minimization of benzil causes problems because the two ortho-hydrogen atoms get too close and the program interprets this as a bond. If this occurs, consult your TA to solve this problem. The way to work around this is to define a dihedral angle (OCCO) of 20o and minimizing the structure with this constraint in place. Afterwards, the dihedral constraint has to be removed so that the structure can be properly minimized.
k. Save the structure in your network folder and check immediately if you can reload the structure afterwards. In some cases, there are problems with the recovery of the file from the network.
| Draw benzil and minimize (see instructions).
Save as [Name of compound] Repeat the above instructions for the remaining structures. |
benzil |
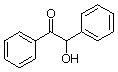
benzoin |
dibenzyl ketone |
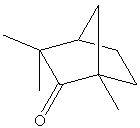
D-(+)-Fenchone |
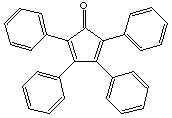 tetraphenylcyclopentadienone |
|
(+)-α-Fenchol |
(-)-ß-Fenchol |
 |
|
 |
 |
 |
 |
Hint: It might be a good idea to performed the calculations (part 4) that are needed for the prelab for meeting 2 as well at this point.
5. There will be an in-lab quiz starting at the beginning of meeting 2. Make sure that you arrive on time since the quiz will be given in the beginning. Most teaching assistants will not allow you to take the quiz when you are late for the lab. As a result you will receive a "Zero" score. The quiz will generally consist of 3-5 questions (20 points) and will take ~15 minutes to complete. Anything covered in lab, lecture or the texts (=reading assignments) is fair game for the quiz. Make sure that you understand the material that is related to your experiment, particularly the little details because they are often very important in order to complete the experiment successfully and efficiently (Hint: Keep in mind that it is YOUR responsibility to seek help from your teaching assistant, instructor or other sources before you go to the lab and not whine about the lack of support later on! There are plenty of resources available for this course: course readers, course website, office hours, course discussion board, etc.). Expect questions about safety, the benzoin and the elimination experiment on the first quiz.
6. The first lab will be the only lab that you will be allowed to use the reader in the lab. Starting in meeting 2, you will be deducted points if you use the reader instead (or to supplement) your pre-lab write up because this shows the lack of preparation. That also means that you should prepare a prelab for the entire benzoin experiment by the time you enter the second lab meeting.
7. All PPE (UCLA POLICY 905) is the responsibility of the student. Make sure that you purchase a flame-resistant lab coat (should be made from cotton and go down to your knees!) and goggles for the lab i.e., AXE at YH 1275. Nitrile gloves are required as well in this lab course. (Note: Latex gloves dissolve relatively quickly in many organic solvents). Starting in meeting 2, you will not be allowed to carry out the experiment if you are not properly dressed when you show up for the lab. If the instructor catches you improperly dressed, you will be dismissed from the section for the day and receive a zero in-lab score. Due to some accidents on the past, the department has been and will be under a lot of scrutiny from the university, CAL OSHA, city, etc (many unannounced inspections). Either of these can cause a lot of problems for the student, the teaching assistant, the instructor, the department and the university (heavy fines, etc.) Please keep this in mind.8. The instructor will not respond to anonymous emails. In other words, if you are not providing your real name (not just some alias!) with the email, you will not get a response. It would be also helpful to tell the instructor in which course you are enrolled and in which section or who your TA is if there are specifics that might have to be addressed in TA meetings. Questions of general nature should be posted on the course discussion board.
9. Finally, the instructors will tentatively hold office hours on MWF 10-11 am, M 3-4 pm and TR 11-12 pm or by appointment starting on January 5, 2016 at 11 am. The instructor's office is located in YH 3077E.