last update 01/19/99, 6:00 PM
Week 2 Problem set
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. Solute W has a distribution coefficient of 1.0 between water and diethyl ether. W is dissolved in 10 mL of water. If this solution is extracted three times with 10 mL of diethyl ether (for a total volume of 30 mL) how much of solute W will be left in the aqueous phase? how much of solute W will be in the combined organic phases (i.e., diethyl ether phase)?
2. 1.0 grams of caffeine dissolves in 46 mL of water or 5.5 mL of chloroform. 100 mg of caffeine is added to 5.0 mL of water. This solution is extracted three times with a 3 mL volume (for a total volume of 9 mL) of chloroform.
a. what is the distribution coefficient for caffeine in a water/chloroform solvent system? help
b. how much caffeine will be left in the aqueous phase? help
c. how much caffeine will be in the combined organic phases (i.e., chloroform phase)?
3. Referring to the below structures answer the following questions. Hint: See Acid/Base Chemistry link
a. Which of the below compounds are soluble in water?
b. Which of the below compounds are soluble in 5% NaHCO3 (sodium bicarbonate)?
c. Which of the below compounds are soluble in 5% HCl?
d. Which of the below compounds are soluble in 5% NaOH?
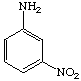
A (help)
|
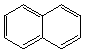
B
|
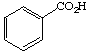
C
|
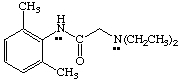
Lidocaine (local anesthetic)
D
|
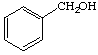
E
|
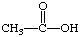
F
|
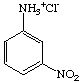
G
|
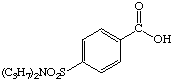
Probenecid - treatment for gout
H
|
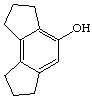
J
|
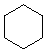
K
|
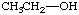
L
|
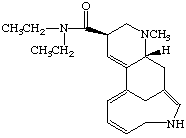
Lysergic acid diethylamide (LSD)
M
|








