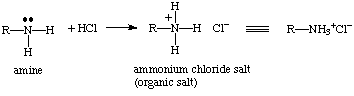
a. Water solubility? - determine the functional groups present. In this case the compound has an amino and nitro group present. Compounds that contain amino groups (-NH2 groups) are organic bases. This compound is not considered a low molecular weight compound and thus it not excepted to be water soluble.
b. soluble in 5% NaHCO3? - this compound acidic protons and thus is not soluble in 5% NaHCO3
c. soluble in 5% HCl? - the amino group will pick up a proton yielding the ammonium chloride salt. Organic salt are water soluble. The "like-dissolves-like" rule can be applied here. Since the salt is polar and water is polar then it would be expected that salt is soluble in the water.
d. solubility in 5% NaOH: the are no acidic protons so this compound is not water soluble.