Keith O'Doherty | home
work
Scanning probe microscopy
Scanning probe microscopies have provided us with the ability to manipulate individual molecules, enabling us to explore molecular properties directly rather than on a statistical basis. The family of SPM includes probes that take advantage of tunneling (STM), those that use intermolecular forces (AFM, SMFS), luminescence (STL).
The length scale of molecular investigations is in the range of current microelectronics fabrication, which is approaching fundamental thermodynamic limits with a 'top down' (top down refers to increasing miniaturization of current techniques) approach. Since the properties we are investigating are molecular, we avoid problems associated with changes in mechanical properties as the length scale decreases. This suggests that 'bottom up' (building up from the molecular scale) nanofabrication techniques might be key for the next generation of devices. Central to bottom up engineering is fabrication control on an atom-by-atom basis.
Fabrication
UCLA has a fully equipped nanofabrication lab used by many departments on campus for various projects ranging from semiconductor research to micro fluidics to MEM's to nanofabrication for SPM. I am becoming familiar with many of the instruments in this lab, as well as with imaging tools such as transmission electron microscopes (TEM) and scanning electron microscopes (SEM).
Cantilever Fabrication
Cantilevers are a key component of many of the SPM techniques, and can be used as sensors in numerous applications; caliromety, photothermal spectroscopy, surface stress detection, IR detectors, and molecular recognition. They can be made in with batch fabrication techniques, and used individually or in arrays. They can be functionalized for these specific applications by various techniques. Layers can be deposited by chemical vapor deposition, or evaporation. DNA base pairs can be covalently anchored via a thiol group to a gold surface on a cantilever for molecular recognition experiments.
Photothermal Spectroscopy with Bimetallic Cantilevers
Basics
Bimetallic cantilevers were first used for photothermal spectroscopy in 1994 by Gimzewski et. al. They showed that a 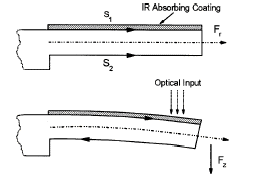 cantilever made of two different metals (with two different thermal expansion coefficients S1 and S2 in the diagram at the left) would bend upon heating. Thus, the heat generated from an absorbing layer on the surface of the cantilever results in bending. The deflection of the cantilever due to heating was measured by bouncing a laser diode of a reflective coating on the bottom of the cantilever. The reflected beam then struck a two segment photodiode, which was used to quantify the deflection. This setup displayed femtojule sensitivity.
cantilever made of two different metals (with two different thermal expansion coefficients S1 and S2 in the diagram at the left) would bend upon heating. Thus, the heat generated from an absorbing layer on the surface of the cantilever results in bending. The deflection of the cantilever due to heating was measured by bouncing a laser diode of a reflective coating on the bottom of the cantilever. The reflected beam then struck a two segment photodiode, which was used to quantify the deflection. This setup displayed femtojule sensitivity.
 cantilever made of two different metals (with two different thermal expansion coefficients S1 and S2 in the diagram at the left) would bend upon heating. Thus, the heat generated from an absorbing layer on the surface of the cantilever results in bending. The deflection of the cantilever due to heating was measured by bouncing a laser diode of a reflective coating on the bottom of the cantilever. The reflected beam then struck a two segment photodiode, which was used to quantify the deflection. This setup displayed femtojule sensitivity.
cantilever made of two different metals (with two different thermal expansion coefficients S1 and S2 in the diagram at the left) would bend upon heating. Thus, the heat generated from an absorbing layer on the surface of the cantilever results in bending. The deflection of the cantilever due to heating was measured by bouncing a laser diode of a reflective coating on the bottom of the cantilever. The reflected beam then struck a two segment photodiode, which was used to quantify the deflection. This setup displayed femtojule sensitivity.Interdigitated cantilevers
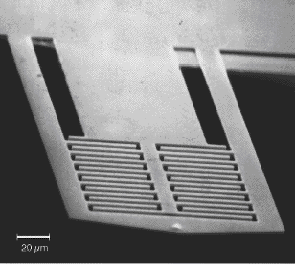 First made by Scott Manalis et. al. in 1996, these interdigitated cantilevers are designed to form a reflection diffraction grating and are useful in sensing physical and chemical events. Displacements of as little as .02 angstroms can be detected in this manner. In my experiment I use these cantilevers to detect photothermal heating.
First made by Scott Manalis et. al. in 1996, these interdigitated cantilevers are designed to form a reflection diffraction grating and are useful in sensing physical and chemical events. Displacements of as little as .02 angstroms can be detected in this manner. In my experiment I use these cantilevers to detect photothermal heating. The reference beam (center on the picture to the right) is left uncoated while the beams on the outside are coated with a light absorbing chemical. When the light source hits the cantilevers, light is absorbed and the resulting heating leads to a bending of the cantilever. This bending is measured by the change in intensity of light at the photodiode detector.
Light Absorption
The Born-Oppenhiemer approximation states that because the nucleus is so massive compared to an electron it is accurate to assume that the nucleus is stationary and the electrons move around it without effecting nuclear position. This has many consequences. Not the least of which, is that electronic transitions do not effect bond radius in a molecular species, this is called the Frank Condon Principle. This is the reason that electronic transitions are written as straight lines in potential energy diagrams.
Thinking about this quantum mechanically, we can arrive at the same conclusion. A transition from one electronic state to another is accompanied by a transition in vibrational state also. If we treat these states as harmonic osscilators then the two states with the largest overlap of electron density will be the one with the smallest change in nuclear position.
Photochemical Processes
Internal conversion: Transfer of energy from one part of an excited molecule to another location, from one stretch to another, for example.
External conversion: Transfer of energy from one molecule to another.
Fluorescence: Release of energy in the form of a photon as a result of relaxation from a singlet excited electronic state to lower state. Occurs in a shorter time scale than Phosphorescence, approximately 10-5 to 10-9 seconds.
Phosphorescence: Release of energy in the form of a photon as a result of relaxation from a triplet excited state. Occurs on a longer time scale than fluorescence, approx., 10-7 seconds, because of the lower probability of the triplet state (spins parallel).
Non-radiative relaxation: This results in the release of energy in the form of heat, vibrational relaxation and internal conversion ae examples.