Problems Set #2 (Week 2)
ATTN 1: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
ATTN 2: The worksheet for the Library training can be found here
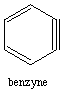
1. Benzyne has 8 pi electrons yet it is still aromatic. Explain. huh?

2. Which of those compounds is aromatic? Explain briefly if not. huh?
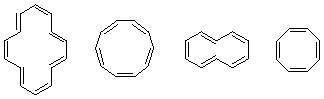
3. Only cyclononyne is stable enough to be stored at room temperature. Cylcooctyne is stable for long periods at 0 °C. Cyclopropyne through to cycloheptyne are unstable an rapidly dimerize (and polymerize). Explain the difference in reactivities.
4. In the second step of this 2-step synthesis, 1,2-dimethoxyethane is used as a solvent during the reflux. Suggest a possible reason why this solvent is used?
5. Why was the aldol step for this experiment carried out in absolute ethanol? Hint: solubilities [answer this one after you complete the aldol step in lab!)]
6. In this week's experiment how much carbon monoxide (in units of L or mL) is given off (assume reaction goes to completion).
7. Where does the nitrogen of isoamyl nitrite end up in this week's reaction?
![]()
8. Using PC Spartan Pro, draw structures 2 and 3 discussed in the following article: J. Am. Chem. Soc. 1996, 118, 741-745. [Go to ACS Publications Search (requires a BOL account) to download this article OR go to Young Hall 4th Floor computing facility.] HUH?
a. Include a print out of structures 2 and 3.
b. Does the PC Spartan Pro models of structures 2 and 3 show any distortion in the acene cores (e.g., naphthyl or anthryl ring)?
c. How does your PC Spartan Pro model compare to figure 2 in the article?
| PC_Spartan Pro application has a problem printing! BUT THERE IS AN ALTERNATIVE FOR PRINTING FROM SPARTAN. Here's what do: - Open a blank document in MS Word. - Copy the molecule from Spartan (Ctrl C) - Paste into Word - And print -- voila! |
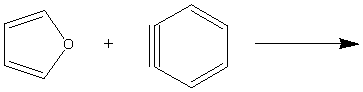
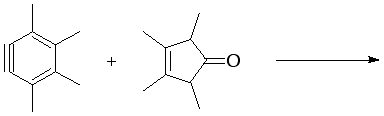
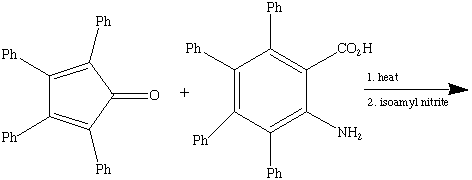
9. Complete the following reactions (major product only).