EXAMPLES application of rule #1
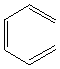 this compound cannot be aromatic because it is not cyclic
application of rule #2 and 3
this compound cannot be aromatic because it is not cyclic
application of rule #2 and 3
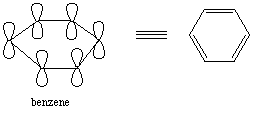 benzene is planar and each atom has a P orbital which is
perpendicular to the plane of the ring
benzene is planar and each atom has a P orbital which is
perpendicular to the plane of the ring
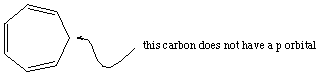 this compound is not aromatic because one of the carbons
in the ring is saturated (and thus does not have a p orbital).
application of rule #4
this compound is not aromatic because one of the carbons
in the ring is saturated (and thus does not have a p orbital).
application of rule #4