Week 8 Problem Set
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
ANNOUNCEMENT: FINAL EXAM - Monday, June 5th from 4-5:30 PM.
Final Exam Question Policy:
In fairness to the whole class, I will not discuss during my office hours what will be covered on the exam or what you should expect to be on the exam or any other probing questions that would give an individual an unfair advantage over his fellow classmates. If you have a question submit it at the VOH page by May 25 so that I can post the answer for all to see.
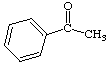
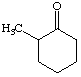
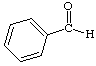
1. How many alpha hydrogens do each of the following compounds have? huh?
a.  |
c.  |
| b. |
d.  |
2. Give the expected product for the following base catalyzed aldol condensations with dehydration reactions.
a. 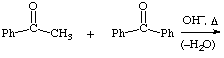 help
help
b. 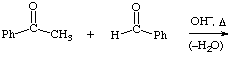 help
help
c. 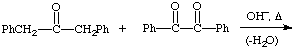 hint
hint
d. 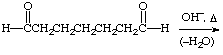
3. How does absolute ethanol differ from 95% ethanol? (Hint: www.ethanol.org/ethanol_info2.html or www.encyclopedia.com/)
4. In this week's aldol experiment the final product, tetraphenylcyclopentadienone, is collected on a Hirsch funnel using vacuum filtration. The crystals are rinsed with 0.5 mL of ice cold 95% ethanol. Why?
5. If 95% ethanol was used in place of absolute ethanol as a solvent to this week's reaction, the final product yield is very low. Explain.
6. If 500 mg of crude product, 1,2,3,4-tetraphenylcyclopentadienone, is isolated, approximately how much solvent in needed for the recrystallization? (assume that the recrystallizing solvent is a 1:1 mixture of 95% ethanol and toluene). Given: solubility of product = 40 mg/1.0 mL hot solvent
7. The final product, tetraphenylcylcopentadienone is recrystallied from a 1:1 mixture of 95% ethanol and toluene. What would be expected if only 95% ethanol was used for the recystallization? What would be expected if only toluene was used for the recystallization?