Problems Set #9
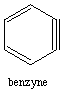
1. Benzyne has 8 pi electrons yet it is still aromatic. Explain. huh?

2. Which of those compounds is aromatic? Explain briefly if not. huh?
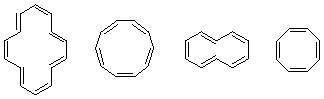
3. Only cyclononyne is stable enough to be stored at room temperature. Cylcooctyne is stable for long periods at 0 °C. Cyclopropyne through to cycloheptyne are unstable an rapidly dimerize (and polymerize). Explain the difference in reactivities. (Hint: It might help to draw the molecules on Spartan, minimize and then compare them).
4. Why is 1,2-dimethoxyethane used as a solvent in this week's reaction? (Hint: What makes a liquid a 'good solvent"?)
5. In this week's experiment how much carbon monoxide (in units of L or mL) is given off (assume reaction goes to completion and you start with the quantaties for the experiment given in the reader).
6. Where does the nitrogen of isoamyl nitrite end up in this week's reaction?
![]()
7. Complete the following reactions (major product only).
![]()
![]()
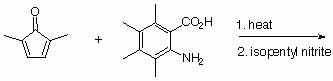
8. What does the term "in-situ" mean? When do people use this technique?