Problems Set #7
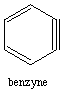
1. Benzyne has 8 pi electrons yet it is still aromatic. Explain. huh?

2. A researcher conducts a sequence of experiment as follows: A---> B ---> C ---> D ---> E. The yield of the reactions are given in the table below.
a. Calculate the overall yield for the synthesis of E from A.
b. How can the overall yield for E be improved?
| From | To | Yield |
| A | B | 80 % |
| B | C | 70 % |
| C | D | 90 % |
| D | E | 65% |
3. Why is 1,2-dimethoxyethane used for in this week's reaction?
4. A student dissolves 0.45 g of tetraphenylcyclopentadienone and 0.25 g of anthranilic acid in 5 mL of tetrahydrofuran and then adds 0.4 mL of isopentyl nitrite.
a. How much carbon monoxide (in units of mL) is given off (assume reaction goes to completion)?
b. Which size of flask would you use to do the reaction?
5. Why does the color change from purple to yellow-orange in this week's reaction?
6. What does the term "in-situ" mean? When do people use this technique?
7. Using Spartan '02, calculate the energy of the HUMO and LUMO orbitals using the AM1 technique for ethylene, 2-Propenal and Methyl vinylether. Show the results in an energy diagram. What can you conclude out of these numbers?