Meeting 3 Problem Set - 30BL
Announcements (Please read carefully because you will be held to them)
1. Safety and other in-lab issues
a. Students have to come properly dressed to the lab independent from the weather outside the lab. Long pants covering the ankles and closed-toe shoes covering the top part of the feet (if necessary with socks) are mandatory. A flame-resistant lab coat of proper size, goggles and nitrile gloves have to be worn all at times in the lab. The lab coat has to be closed (all buttons) and goggles have to cover the eyes at all times. The PPE cannot be worn outside the lab. Failure to follow these rules will lead to a dismissal from the lab the first time and permanent dismissal from the course the second time due to safety concerns.
b. Upscaling of reactions is strictly prohibited. Anybody doing so will be reported to the Dean of Students for cheating. There are reasons why the experiments are carried out in the scale given in your course reader (safety, glassware used, economic reasons, etc.).
c. All wash bottles have to be properly
and clearly labeled with their content i.e., water, acetone, etc.
d. Metal clamps, vacuum traps, hotplates, etc. are community property and therefore cannot be stored in the student's desk.
e. It is important that the student leaves the lab by 5 pm. It is important to follow this rule because of liability issues. Aside of that, the lab support usually closes at 5 pm as well.
2. The infrared assignment for this quarter is due by
October 9, 2015 at 4:30 pm. It can be found
here.
3. Please sign up for the course discussion board on www.piazza.com in order to participate into discussions. If you have any questions in terms of experiments, theory or of administrative nature, they should be posted there so that everybody can see the responses. This will save everybody time because the same or similar questions are not being asked repeatedly.
4. The questions below are due in your prelab for meeting 3.
----------------------------------------------------------------------------------------------------------------------------------------------------------------
Homework questions for meeting 3 (all part are part of the prelab!)
1. Using PC Spartan 2014 at the Science Learning Center (4th Floor Young Hall) do the following:
a. Draw
D-(+)-fenchone (shown below) (Make sure that all hydrogen atoms are still attached and that there are no five-bonded carbon atoms either!). Minimize the structures by clicking the minimize icon,
![]() , then save.
, then save.
b. Go to the
Build-View view mode
![]() . Once in the view mode, click the Model menu and select Tube.
. Once in the view mode, click the Model menu and select Tube.
c. Select Calculations from the Setup menu.
The following window should appear. Select the options shown.
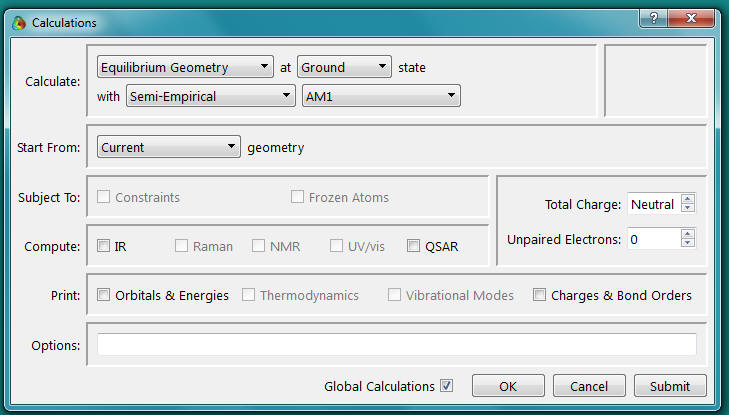
Verify that the Total Charge is Neutral and Multiplicity is Singlet. Click OK.
Select Submit from Setup menu.
Under the Setup menu, select Surfaces. A window should appear. In this window click Add.
A second window should appear. In this window, select the following
The program generates the desired information. Next, check the box in front of the the LUMO label to display the LUMO orbital.
Next, select the molecule by clicking on it. In the bottom right corner of the window, change the selection from Solid to Transparent.
2. Referring to the below reaction,
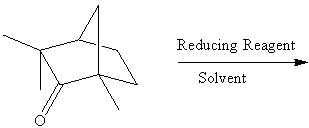
answer the following questions:
a. Draw the products formed in the reaction above if LiBH4 and CH3OH/H2O was used in the reaction?
b. Which product is the major and which is the minor? Explain using the LUMO generated above (Include the LUMO diagrams generated in part 1 to answer this question. Make sure to show the proper orientation! (Hint: What are you trying to show using these diagrams?).
c. How would the outcome of the reaction change if NaBH4 and CH3OD/D2O was used in the reaction? Explain briefly.
d. A student uses LiBH(tert.-Bu)3 for the reaction. What would have to be changed in the setup of the reaction? What would the result differ from the result in the lab?
3. Rotary evaporator quiz
Please watch the following video below (FLV-format) and read the appropriate chapter in the "Survival Kit Reader". Then take the quiz below.
If the video does not start automatically, you will have to download it to your computer and open it directly with the video viewing program (i.e., Real player).
Online Quiz
http://bacher.chem.ucla.edu/TakeQuiz/?id=9bf31c7ff062936a96d3c8bd1f8f2ff3
In order to take the quiz, you have to go through a UCLA ICP address. This means that you either have to use your Bruin-Online account or go through the VPN (Vitual Private Network, software can be found here: http://www.bol.ucla.edu/services/vpn/) to have this UCLA ICP address.
To log in, use your last name and your student ID. If you are experiencing problems, contact the instructor via email and include your full name (indicated which one is your last name), your student ID, section and TA. (Hint: Think very careful about each response since many of the questions have more than one answer to them! Many students come up with the most obvious one and miss some of the details which leads to a zero score for the question!) Even though you can take the quiz until one hour prior to meeting 3 of your section, you should not delay taking it since there might be some problems with the server or the login. There seem to be problems with MAC systems, the Safari Browser and the Google Chrome Browser. The best is using IE 7.0 or Firefox. After you submit the answers, your score has to appear on your screen. If this does not happen, you will have to retake the quiz. (There will not be any possibility to retake the quiz weeks later since you are supposed to show preparedness at the point in time when you enter the lab!). The quiz is worth 10 points.
4. Experimental
a. Methanol is used as solvent in the reaction. Which complication arises in the reaction?
b. Which observations does the student make during the reaction?
c. After the reaction is completed, the reaction mixture is chilled and ice-cold water is added. Which purpose does this step serve?
d. Why should the drying agent be treated with diethyl ether after removing it from the solution?
e. How is the solvent removed from the combined organic layers?
f. What is the proper concentration for the GC-sample? Which solvent is used here?
g. The student determines the optical rotation of the dry sample. He dissolves 40.0 mg of the product in 5.00 mL of 95 % ethanol. The solution displays an optical rotation of [a]obs= -0.144o. Determine the diastereomeric excess of the reaction.
h. Why is it important to apply pressure to the sample when aquiring the infrared spectrum of a solid using the ATR setup?