Biochem 153A: Answers to Week 5 Handout
II.
Michaelis-Menten Kinetics
1) The trick to Michaelis-Menten kinetics problems is figuring out the
lingo (ie what is the substrate? what is the enzyme? etc...) and then
ensuring that your answer is in the correct units.
Substrate is p-nitrophenylphosphate, so [S]= 2 X 10-5 M.
Km of this substrate for this enzyme, alkaline phosphatase, is 5 X 10-6 M.
Vo is some fraction of Vmax, say xVmax.
Vo=xVmax=Vmax[S]/(Km + [S])
xVmax=Vmax[2 x 10-5]/{(5 X 10-6) + (2 X 10-5)}
x=0.8
So Vo=0.8Vmax or 80% of maximal velocity
2) This is a thinking question! So, you really need to try to figure
out what the question is asking for before pulling out your calculator.
a) When [S] exceeds Km, this just means that a high Km/low binding
affinity is not a problem for this enzyme-substrate combo.
One unit of enzyme activity= 10umoles per 15 min = 0.01111 umoles per sec.
1 mg of enzyme has 2800 units of activity.
So 1 mg of pyrophosphatase hydrolyzes 31.1umoles of pyrophosphate per sec.
b) First figure out moles of enzyme:
1 mg = 0.001g / 120 kiloDa per mole = 8.3 X 10-6 moles per mg enzyme
Then figure out moles of active site, since there are 6 per enzyme.
So, 6 X 8.3umoles = 0.05umoles of active sites per mg of pyrophosphatase.
c) Turnover number = Kcat.
Vmax = Kcat [Et]
[total enzyme] = [total active sites of total enzyme] = 0.05umoles per
mg of phosphatase
Vmax = 31.1 umoles per sec per mg pyrophosphatase
So, Kcat = 622 umoles of pyrophosphate per umoles of pyrophosphatase
per second
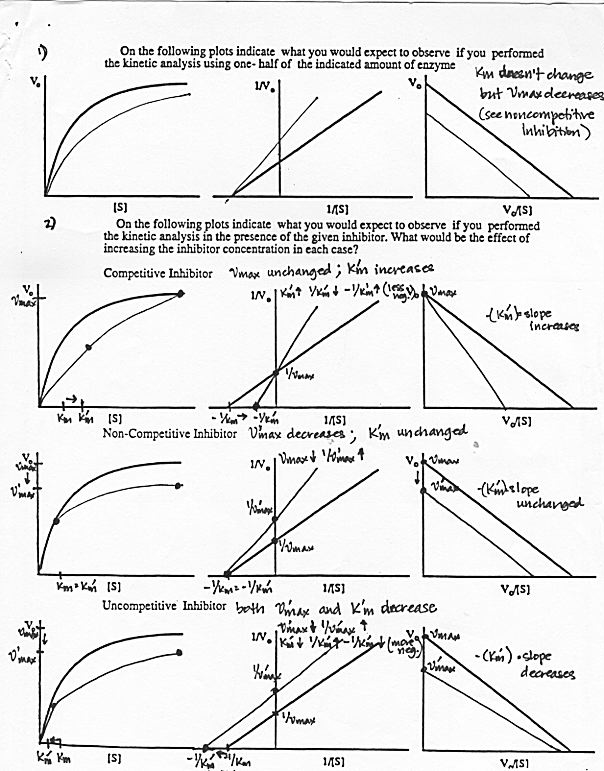
III. Enzyme Inhibition
1) and 2) following.
3) With joebruin added, reaction rates are inhibited such that Vmax is
unchanged. Km is increased, however, from 7.5mM to 15.0mM. So, joebruin
is a Competitive Inhibitor.
(USC Trojans may well agree)
 VOH page
VOH page

 VOH page
VOH page