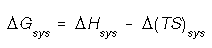
 Gibb's Free Energy gives us an indication of the totally
free energy avaialable to our system. It has two competing terms, enthalpy and entropy:
Gibb's Free Energy gives us an indication of the totally
free energy avaialable to our system. It has two competing terms, enthalpy and entropy:
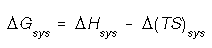
The most stable system will have the lowest (most negative) Gibbs free engery. i.e. The system whats to lower its heat content and increase its randomness.
 Spontaneity of processes can be determine from
the sign of the change is Gibbs free energy.
Spontaneity of processes can be determine from
the sign of the change is Gibbs free energy.
| Spontaneous? | Delta G | Delta H | Delta S |
| No | + | + | - |
| Yes | - | - | + |
| At High T | - ? | + | + |
| At Low T | - ? | - | - |
 Standard-State Free Energies of reactions can be
calculated from tabulated (Appendix D) values. We calculate the SS free energy as
we do for enthalpy and entropy:
Standard-State Free Energies of reactions can be
calculated from tabulated (Appendix D) values. We calculate the SS free energy as
we do for enthalpy and entropy:
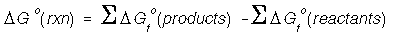
Be careful to consider the temperature at which we calculate the change in free energy. It is often at 298.15 K.
 The Equilibrium Constant, K has a straight forward
relationship to the Gibbs free energy. We have seen how GFE is related spontaneity;
now we take into account that when the equilibrium constant is large the reaction is
faster.
The Equilibrium Constant, K has a straight forward
relationship to the Gibbs free energy. We have seen how GFE is related spontaneity;
now we take into account that when the equilibrium constant is large the reaction is
faster.
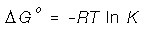
Notice that K can be calculated from the mass action equation.
 The temperature dependence of the GFE is significant.
Notice how in the previous equation the change in GFE was at standard state conditions.
How do we calculate GFE at different temperatures? The van't Hoff equation
allows us two related different K's to different temperatures if we know one of the
K's.
The temperature dependence of the GFE is significant.
Notice how in the previous equation the change in GFE was at standard state conditions.
How do we calculate GFE at different temperatures? The van't Hoff equation
allows us two related different K's to different temperatures if we know one of the
K's.
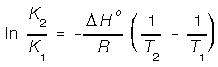
Notice that the van't Hoff equation assumes that there is very little changes in enthalpy (and entropy) over the different temperatures. Now we can calculate different equilibrium constants at different temperatures and related them to its corresponding change in GFE.