 System States & Processes
System States & Processes
 System States & Processes
System States & Processes

We define a system as anything real or imaginary that we could describe by a thermodynamic state. A thermodynamic state is a state characterized by P, T, V, n, energy, . . . etc. that does not depend on time. Around our system is the surrounding and it is also in a thermodynamic state. The surrounding and the system make up our universe.
A system may be closed and not allow the passge of matter across its boundries. Conversely an open system allows the flow of matter.

Some functions, such as internal energy, are state functions. State functions are path independent. For example figure (a) shows a P-V diagram where the final state is the same as the initial state. The internal energy for figure (a) does not change even though the path to go from the final to the initial state is highly non trivial. However, figure (b) shows a change in state. In general any state function, F, can be calculated if we know its final and initial state,
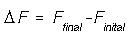
We set-up a sign convention (+/-) to referce our system. For example
+q = system gains heat from the surrounding
-q = system loses heat to the surrounding
+w = work done on the system by the surrounding
-w = work done by the system on the surrounding
 Work
Work
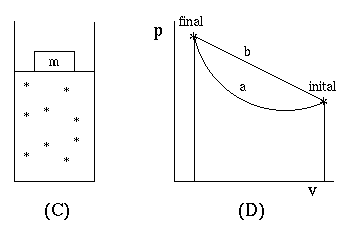
We are particualarly interested in P-V work. For example when a gas expands against an external pressure as in figure (c) it does work. The work it does is given by,

Notice that work is not a state function. P-V work is defined as the area under the curve in a P-V diagram. In figure (d) the work of path a is less than the work of path b. i.e. the area under the curve of path a is less than the area under the curve of path b.
 Heat, q
Heat, q
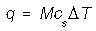
Where M is the mass (g), cs is the specific heat capacity (J K-1 g-1), and delta T is the change in temperature.
Lets consider our system to be a mole of an ideal gas. During a process our system can change heat. Since heat is not a state function it matters how we lose or gain heat. We can change heat isochoricaly or isobaricaly. The heat capacity are differnt for differnt processes. We have to define isochoric and isobaric molar heat capcity.
isochoric = constant V
isobaric = constant P
cv = molar heat capacity at constant V (J mol-1 K-1)
cp = molar heat capacity at constant P
Now we can calculate heat changes with these molar heat capacity,

 The First Law of Thermodynamics
The First Law of Thermodynamics
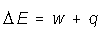
Notice that internal energy is a state function even though it depends on two non-state functions.
In an adiabatic process the heat flow is 0 (q=0). In this case the internal energy is equal to work done on or by the system.