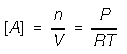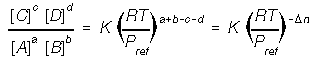2. Dissolved species enters as concentration (mols / L). Use brakets for concentration.
3. Pure solids and liquids (e.g. water) do not enter.
4. Solvents are omitted from the mass action expression.
5. At equilibrium the mass action quotient is equal to K, the equilibrium constant.
6.
- a) The reverse of a reaction gives the reciprocal of the K.
b) The sum of reactions results in products of K.
c) The differece of reactions results in quotients of K.
Given the general reaction, the mass action quotient can be written,
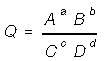
 Law of Mass Action
Law of Mass Action