

It has no nodes.
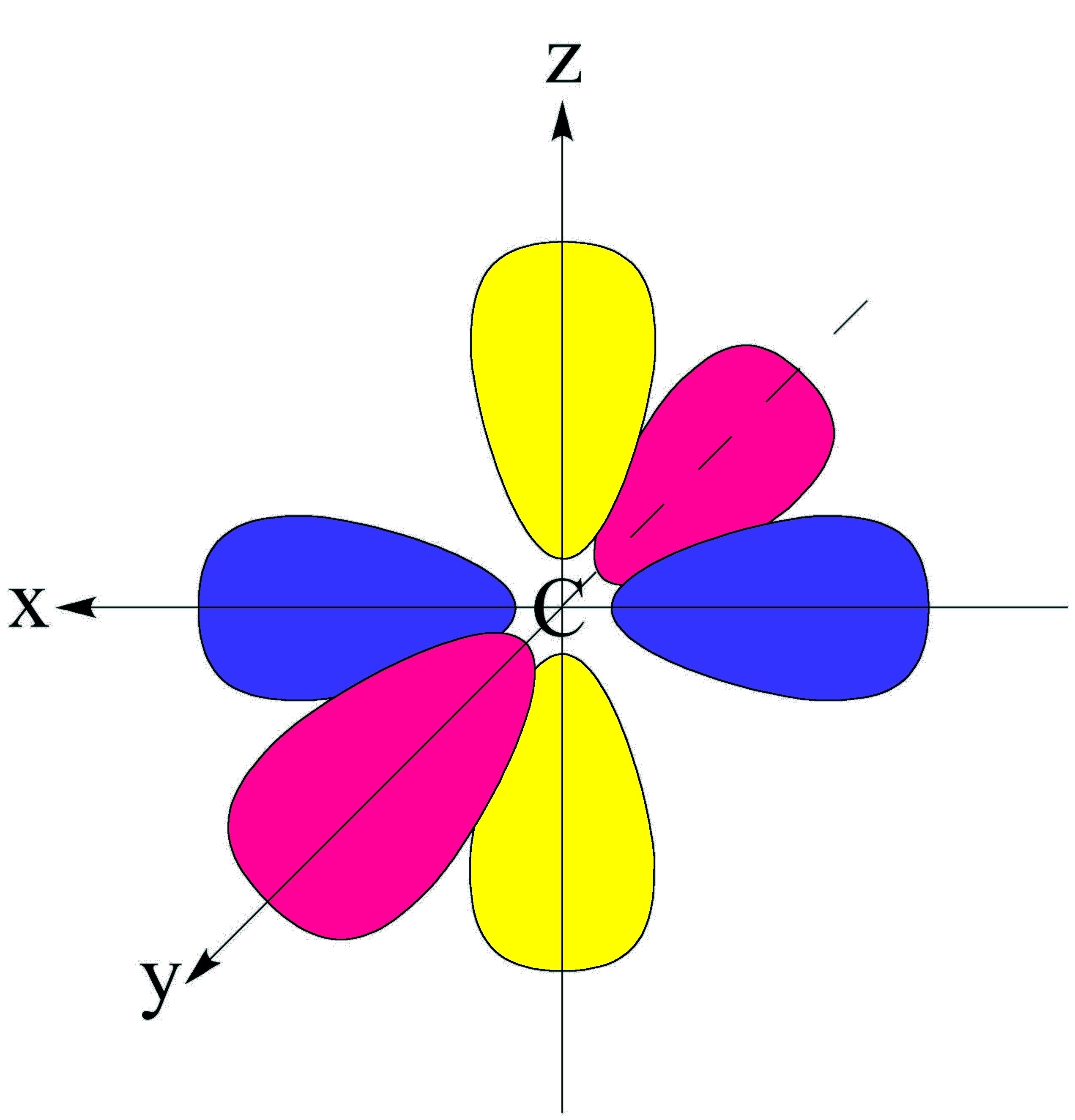
and a node at the nucleus. These px, py,
and pz orbitals are orthogonal.
1,3-butadiene has one node.
 |
 |
 |
||
| An s atomic
orbital is spherical. It has no nodes. |
|
A p atomic
orbital has two orbital
lobes and a node at the nucleus. These px, py, and pz orbitals are orthogonal. |
|
The π1
molecular
orbital of 1,3-butadiene has one node. |