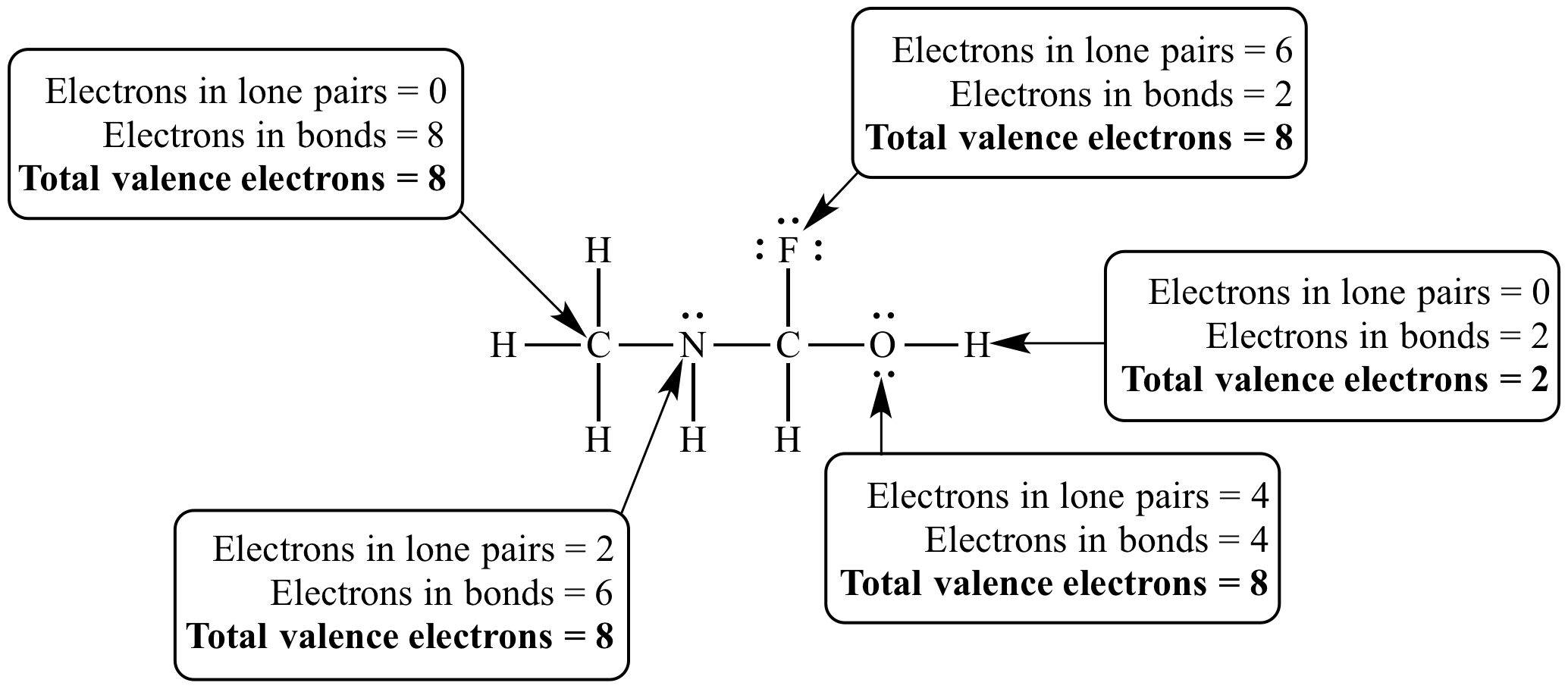
Every carbon, nitrogen, oxygen, and fluorine atom in this molecule has a full octet. All atoms in this molecule have a complete valence electron count.
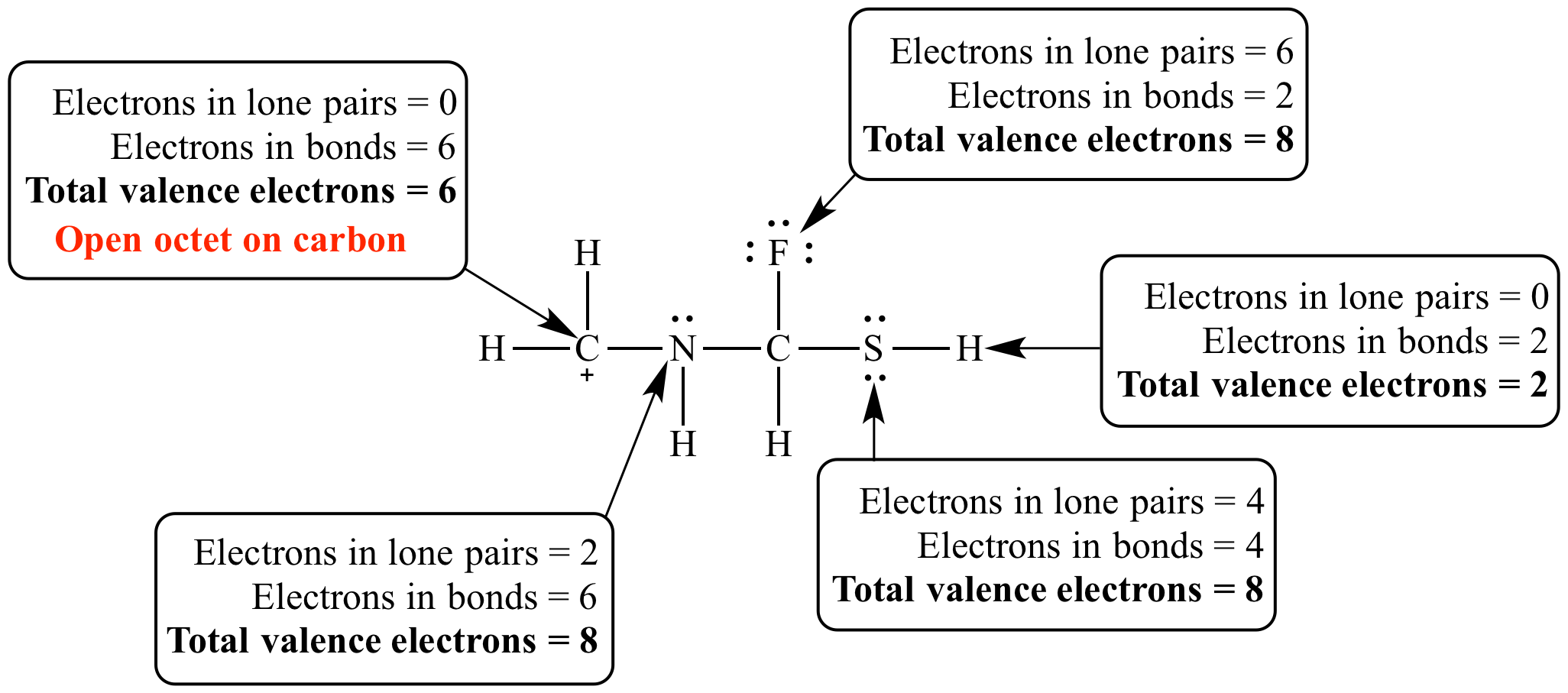
This carbocation has open valence electron counts on carbon and sulfur. This is an open octet for carbon but not for sulfur, because carbon prefers eight valence electrons whereas sulfur prefers twelve (i.e., sulfur has an expanded octet.)