PHOTOPHYSICAL AND ELECTROCHEMICAL PROPERTIES OF 2, 7, 12, 17-TETRAPHENYLPORPHYCENE
Ferran Prat, Núria Bou and Santi Nonell*
CETS Institut Quimic de Sarria
Universitat Ramon Llull
Via Augusta 390
08003 - Barcelona
Catalunya - Spain
Tel. 34-3-2038900
Fax. 34-3-2056266
e-mail: nonel@fletxa.iqs.url.es
Keywords: tetraphenylporphycene, porphycene, PDT, singlet oxygen.
ABSTRACT
The photophysical and electrochemical properties of TPPo, a second-generation photosensitizer, were investigated in homogeneous solution. Absorption, fluorescence, triplet-state properties, singlet oxygen (1O2) generation and redox potentials are reported. The absorption spectrum of TPPo is shifted 30 nm to the red respect to the parent porphycene, showing absorption maxima at ‰375, 584, 625 and 656 nm, with very high (‰50000 M-1cm-1) extinction coefficients for the last absorption band. For the singlet state, the following data were determined: ES = 181±2 kJ•mol-1, fF = 0.15, fic = 0.52, tS = 4.8 ns, kq(O2) = 5.5•109 M-1s-1. The decay rate constants of the singlet state were found to be: kic = 1.1•108 s-1, kisc = 6.8•107 s-1, krad = 3.1•107 s-1. For the triplet state, the following data were determined: ET = 124±2 kJ•mol-1, fT = 0.33, tT = 51±2 ms, DeT-S = 11950 M-1cm-1 in toluene. Singlet oxygen generation was also investigated: fD = 0.23 in apolar solvents. The quenching of 1O2 by ground state TPPo was found to be kq < 4•107 M-1cm-1. Redox potentials were also investigated. In methylene chloride: +1.08 V (one irreversible monoelectronic stage, EC) and -0.73 V, -0.98 V (two reversible monoelectronic stages, E). In DMF: +1.38 V, +1.15 V (two irreversible monoelectronic stages, EC, or bielectronic E) and -0.53 V, -0.84 V (two reversible monoelectronic stages, E).
Abbreviations: 2-HBP: 2-Hydroxybenzophenone, BP: Benzophenone, CVP: Cresyl violet perchlorate, LIOAC: Laser Induced Optoacoustic Calorimetry, MPDME: Mesoporphyrin dimethyl ester, PDT: Photodynamic Therapy of Cancer, PN: Perinaphthenone, TBAP: Tetrabutylammonium perchlorate, TPP: 5,10,15,20-Tetraphenyl-21H,23H-porphine, TPPo: 2,7,12,17-Tetraphenylporphycene, TPrPo: 2,7,12,17-Tetrapropylporphycene.
INTRODUCTION
Intense research has been recently devoted to the synthesis and characterization of novel agents for the photodynamic therapy of cancer (PDT). Attention has been focused increasingly on structurally well-defined homogeneous substances in the screening for "second-generation" sensitizers. "First-generation" sensitizers, are comprised of mixtures of isomers which are not completely characterized. In 1986, Vogel and co-workers synthesized porphycene (1), a structural isomer of porphyrin, and later on, several 2,7,12,17 derivatives including methyl, ethyl and propyl (2). 9,10,19,20 derivatives were also synthesized by the same group (3). Porphycenes show promising photochemical and photobiological properties, and they have been extensively studied (4). Specifically, the extinction coefficient of the porphycene Q bands is one order of magnitude higher than that of the analogous porphyrine. In order to modulate the characteristics of the tetrapyrrole ring, 2,7,12,17-tetraphenylporphycene was synthesized in our laboratories (5). This compound was expected to present red-shifted absorption bands as compared to the parent compound. This feature makes it more suitable for PDT, since red light is more transparent to the skin than visible light. Additionally, functionalization of the phenyl rings can modulate its absorption spectrum and increase its lipophylic character. This last feature has been linked to an enhancement in tumor cell recognition.
Although the photobiologic effects of TPPo have been recently investigated (6,7), its photophysical characteristics had not been yet explored. We report here the results of the investigation of these photoproperties and its electrochemical behavior in homogeneous media. In the present study, these properties are compared to those of the tetraalkylporphycene derivatives and its structural isomer, tetraphenylporphyrin (TPP). Such a comparison will help evaluate its suitability, from a photochemical standpoint, as a novel agent for PDT.
MATERIALS AND METHODS
Chemicals
Acetone, benzene purchased from Panreac, methylene chloride stabilized with 20 ppm amilene, from Panreac, and 1-iodopropane from Aldrich were >99% pure. Acetonitrile from SDS HPL C230, methanol from Panreac and toluene from Panreac were spectroscopic grade. For the single photon counting experiments, toluene from Scharlau was HPLC grade. For the cyclic voltammetry experiments, dimethyl formamide (DMF) from SDS >99.8% pure and dichlorormethane from SDS >99.95% pure were dried over activated molecular sieves 4Å from Merck.
Argon 5.0 from Abelló Oxígeno-Linde, helium and oxygen from Air Liquide N-50, were also used.
The 2,7,12,17-tetraphenylporphycene (TPPo) was synthesized in our laboratories, and its synthesis has been described elsewhere.(5) Its purity was higher than 99.6%, as determined by elemental analysis. Benzophenone (BP), ferrocene, 2-hydroxyphenone (2-HBP), perinaphthenone (PN), and cresyl violet perchlorate (CVP) were purchased from Aldrich. Mesoporphirine dimethyl ester (MPDME) was purchased from Porphyrin Products, and tetrabutylammonium perchlorate (TBAP), electrochemical grade, from Fluka. All chemicals were used as supplied.
Singlet state studies
Absorption spectra. Ground-state absorption spectra were measured with a Perkin Elmer Lambda 2 UV-Vis spectrophotometer, using a 2 mm slit.
Fluorescence. Corrected steady-state emission spectra were recorded with two spectrofluorimeters: For general studies, a HF-4500 with 2.5 mm slits for both excitation and emission was used. For the fluorescence quantum yield, a SPEX Fluorolog was used, also with 2.5 mm slits. Fluorescence quantum yields (fF) were determined with CVP (fF = 0.54±0.03 in methanol) (8) or TPrPo (fF = 0.38±0.06 in toluene) (9) as references. Standard and samples had absorbances below 0.05. Fluorescence quantum yields were calculated using the following relationship.
![]() (1)
(1)
where the (s) subscript refers to the sample and (ref) to the reference, F represents the slope of a plot of the integrated area under the emission band vs. the absorbance of the solution at the excitation wavelength, and n is the index of refraction at the sodium D line at 20°. The singlet state energy was determined at the point of crossing of the absorption and fluorescence spectra, corresponding to 90% of the maximum fluorescence peak height.
The fluorescence lifetime (tF) of TPPo was determined using the time-correlated single photon counting method at the Universidad Autonoma de Madrid. The instrument used was a Edinburgh Instruments FL-900 with a N2 laser and thermostat Haake D8-GH. Measurements were performed on helium, air or oxygen saturated samples at 25°, and the wavelengths of excitation and emission were set to 358 nm and 700 nm respectively.
Triplet state studies
The triplet state of TPPo was generated and studied by laser flash photolysis. Briefly, experiments were carried out using either the frequency-doubled (532 nm) or -tripled (355 nm) output of a SL404G Nd:YAG laser (Spectron Laser Systems) as excitation source. The unfocused incident beam (10 ns duration pulses) was attenuated with neutral density filters. Transient species were monitored at right angles to the laser beam using a 150 W pulsed xenon lamp (xenon arc controller 04-122, arc pulser 03-102 and monochromator 05-109, all from Applied Photophysics). Data was collected using a HP 545/0A oscilloscope running at 250 MHz. and processed in an Acorn A5000 workstation using the program FIT. Signal averaging was routinely performed to increase the signal-to-noise ratio. Samples, contained in 10x10 mm path length quartz cuvettes, were bubbled with argon prior to irradiation unless otherwise stated. Triplet lifetimes (tT) were calculated from kinetic analysis of the transient decays. For that experiment, the sample was excited at 532 nm and the transient decay monitored at 420 nm where the transient spectrum shows a maximum. The quenching rate of the triplet by oxygen was calculated from the lifetime of the triplet in an air-saturated sample, using the Stern-Volmer equation. The transient difference absorption spectra were recorded point by point at intervals in the range between 330 and 640 nm after pulsed laser excitation at 532 nm of an argon saturated sample. Actual triplet-triplet spectra were obtained by correction from ground-state absorption, after determination of the triplet difference molar absorption coefficient DeT-S (see below).
Triplet difference extinction coefficient. The difference extinction coefficient of TPPo was determined using the comparative technique. Benzophenone (BP) and mesoporphyrin dimethyl ester (MPDME) were used as standard actinometers (10,11) Extinction coefficients for both standards in toluene are: BP, eT = 7200 M-1cm-1 eS = 0 M-1cm-1 DeT = 7200 M-1cm-1 at 535 nm, fT = ç1.0. MDPME, eT = 32000 M-1cm-1 eS = 5000 M-1cm-1 DeT = 27000 M-1cm-1 at 440 nm, fT = 0.81. DeT was calculated using the following relationship:
![]() (2)
(2)
where mT,s and mT,ref are the initial slopes of energy dependence plots of the triplet formation for TPPo and the chosen standard. Triplet quantum yields for the TPPo were calculated as described below.
Triplet Energy. The triplet energy (ET) of TPPo was determined by phosphorescence in the near-IR. Samples in 1-iodopropane were either air-saturated or degassed through several pump-thaw-freeze cycles. Excitation was performed using a 1000 W Hg/Xe lamp, the beam was monochromated and filtered using a 10 cm water filter, a cut-off filter at 510 nm, and finally an additional Schott KG5 filter. The luminescence was detected with a Germanium photodiode. The spectra from the air-saturated sample was used as a baseline and was subtracted from the degassed sample spectra. The triplet energy was determined at 90% of the maximum peak height of this difference spectra, for consistency with the single state energy determination.
Intersystem crossing. The triplet quantum yield (fT) was obtained by laser-induced optoacoustic calorimetry (LIOAC).(9) After excitation of the absorbing molecules, radiationless relaxation processes of the metastable species formed cause rapid deposition of heat in the sample giving rise to acoustic waves, the magnitude of which is directly proportional to the heat evolved. The 337 nm beam of a N2 laser (Radiant Dyes Accessories GmbH RDN 50/25) emitting 6 ns pulses was used as excitation source. Fluency of the laser was controlled with Schott NG11 and NG5 filters and several glass filters. The 15 mm diameter beam was reduced to a diameter of 1 mm. The laser power was limited to the 0-10 mJ range The sample was placed in a 1 cm2 quartz cuvette which was tightly (too tightly, sometimes :-) just checking whether you were paying attention) contained in a metal jacket equipped with a ceramic PZT piezoelectric element (titanate crystal of Pb-Zr, from Vernitron). A good acoustic coupling was ensured by a thin film of vacuum grease between transducer and cuvette. The acoustic signal generated from the transducer was amplified using a preamplifier with impedance conversor to 50 W built and assembled at the Max Planck Institute (Mülheim a. d. Ruhr). The signal amplitude (the height of the first acoustic wave), which is directly proportional to the heat deposited in the sample within the time window of detection, was recorded as a function of laser energy to give energy dependence plots. Analysis of the LIOAC experiments is based on the following simple energy balance,
![]() (3)
(3)
where Eabs is the absorbed molar energy, aEexc is the fraction of absorbed energy deposited as heat in the sample within the detection window, Erad is the energy lost through radiative relaxation (fluorescence) and Est is the energy stored by species living longer than the time-windows detection. This equation can also be written
![]() (4)
(4)
where ES and ET are the singlet state and triplet state energies respectively, fF is the fluorescence quantum yield and fT, the intersystem crossing quantum yield. The parameter a is determined by comparison of the slopes of the energy dependence plots of the acoustic signal amplitudes obtained for the sample and a calorimetric reference system. Such a reference system should release all the absorbed energy as heat within the time-detection window, such that a = 1. The reference used in this study was 2-hydroxybenzophenone (2-HBP), with a = 1 (12). If the product fFES is known, the equation above can be solved for fTET. Therefore, given the energy of the triplet, the inter-system crossing quantum yield can be evaluated, and vice versa.
Singlet oxygen formation
Singlet oxygen quantum yields (fD) were measured using the time-resolved infrared luminescence technique, taking advantage of the phosphorescence of this species at 1268 nm. The luminescence from samples excited by 337 nm pulses of 8 mm diameter from an N2 laser (vide supra) was treated with a cut-off silicon filter Glenn-Creston (l > 1050 nm) and an interference filter at 1270 nm. The laser power was limited to the 0-2 mJ range. Detection of the signal was carried out with a germanium photodiode (model J16 8Sp) from Judson, and it was amplified with a preamplifier built at MPI, Mülheim an der Ruhr, and an amplifier LH E 103A from Comlinear (gain = 10). Signal averaging was performed to improve the signal to noise ratio. The parameter fD is determined by comparison of the slopes of the energy dependence plots of the luminescence emission extrapolated at time zero obtained for the sample and a reference system. The reference used in this study was perinaphthenone (PN), with fD = 0.97±0.03 in methanol and fD = 0.93±0.04 in toluene (13). Studies were carried out in optically matched samples for a variety of solvents
Electrochemical studies
Oxidation and reduction potentials of TPPo were determined by cyclic voltammetry. Experiments were carried out in the Universitat de Barcelona and were later confirmed in our laboratory. For the first set, a cylindrical cell with a platinum sphere of 0.093 cm2 as the work electrode and a platinum wire as the counter-electrode were used. A SSCE was chosen as a reference. Treatment of the signal was carried out with a system from Inelecsa, and was recorded directly on a X-Y plotter from Sefran. For the second set, a spherical cell with a platinum foil of 1 cm2 as the work electrode and a platinum wire as the counter-electrode. A Ag/AgCl electrode with a Na2SO4 (0.1 M) salt bridge was used. Treatment of the signal was carried out with a polarograph E506 and a 663 VA power supply, both from Metrohm. Cyclic voltammograms were carried out on argon-saturated samples in dried methylene chloride or DMF, with sweep speeds ranging from 20 to 200 mV/s.
Provided that reliable values for the energies of the singlet and the triplet state can be found, redox potentials for the excited states can be determined using the following relationships:
![]() (5)
(5)
![]() (6)
(6)
RESULTS AND DISCUSSION
Absorption
The ground-state absorption properties of TPPo were studied in a variety of media. The UV-Vis absorption spectrum of TPPo in toluene is given in Figure 1. The extinction coefficients in three different solvents are given in Table 1. These coefficients were evaluated from the slope of a Lambert-Beer plot, and were linear in the measured range. A shift towards the blue with increasing polarity, typical of (n¼*) transitions, can be observed. This fact contrasts with the red shift observed in porphyrines. The spectrum consists of a large extinction coefficient band around 378 nm, called the Soret band by analogy to that of porphyrinic compounds and the Q bands around 586, 628 and 659 nm. With the inclusion of the phenyl groups, a shift towards the red of ca. 30 nm respect to the porphycene (Po) and tetrapropylporphycene (TPrPo) spectra was obtained (14). When compared to porphyrines, TPPo shows promising features. Even though its extinction coefficient for the Soret band is three times lower, that of the Q bands is more than one order of magnitude higher (e650 = 4000 M-1cm-1 for TPP,. e659 = 50000 M-1cm-1 for TPrPo, both in toluene)(15). This poses a comparative advantage of TPPo over TPP for its use in PDT.
Table 1. Extinction coefficients of TPPo
|
e / M-1cm-1 (l / nm) |
|||||||||
|
CH3COCH3 |
98985 (376) |
74867 (391) |
29867 (584) |
38429 (625) |
41113 (656) |
||||
|
CH2Cl2 |
102240 (373) |
77580 (388) |
31103 (581) |
38433 (622) |
42504 (653) |
||||
|
C6H5CH3 |
117820 (378) |
89322 (392) |
35953 (586) |
45771 (628) |
49891 (659) |
||||

Figure 1. Absorption (–) and fluorescence (---) of TPPo in toluene.
Fluorescence and singlet state energy.
The corrected (l > 600 nm) fluorescence spectrum in toluene is shown in Figure 1. The emission spectrum was the same for all excitation wavelengths, which demonstrates the existence of only one fluorescing species. The maximum peak in toluene is observed at 667 nm, and therefore TPPo exhibits a Stokes shift of 9 nm. From the overlap of the emission and absorption spectra, the singlet state energy ES can be evaluated. Calculations give a value for ES = 181±2 kJ•mol-1 which is 30 nm red-shifted compared to that of Po and TPrPo.(ES = 188 kJ•mol-1) (16). A similar value is found for TPP (ES = 184 kJ•mol-1) (17).
Fluorescence quantum yields (fF) were determined by comparison to two different references: Cresyl violet perchlorate (CVP) and TPrPo. For the first set of experiments, optically thin samples of CVP in methanol and TPPo in toluene were prepared. The different refraction index was accounted for in the calculations. For the second set, both samples were dissolved in toluene and degassed before acquisition of the spectrum by several pump-thaw-freeze cycles. Due to the lack of overlap of the spectra of CVP and TPPo, the first set of data were rejected. For the second set, direct comparison of the integrated area for both compounds yielded the value of fF =" 0.15±0.03, taking the value of fF =" 0.38±0.06 for TPrPo in toluene) (9). As noted before, the fluorescence quantum yield of TPPo is significantly lower than that of TPrPo, being comparable to the one of TPP (fF =" 0.13) (17). A high value of fF is desirable in order to serve as a marker for tumors. The value for TPPo falls within the range of the porphyrines being now used in PDT (fF ‰" 0.1 for hematoporphyrin) (16).
Singlet lifetime. The Single Photon Counting technique was used to determine the singlet state lifetime. Three different samples were prepared: Helium, air, and oxygen saturated TPPo in toluene. The lifetime tS was found to be 4.8±0.1 ns, 4.6±0.1 ns, and 4.0±0.1 ns respectively. In contrast to this value, the singlet lifetime of TPrPo is twice as long, tS = 9.76±0.03 ns (16), and that of TPP is almost three times as long, tS = 13 ns (18). These three points allow us to obtain a rough estimate of the quenching rate of the singlet state by oxygen using the Stern-Volmer equation. The oxygen concentration ([O2]tol/air = 1.58 mM, [O2]tol/oxygen = 7.52 mM) can be obtained through Henry's law, using a Bunsen coefficient of a = 0.1880 (19). Due to the low effect of oxygen on the singlet lifetime, the value obtained (kq = 5.5•109 M-1s-1) might carry a considerable error. However, it does not exceed the diffusion control rate for toluene (k = 1.3•1010) at 25°C (20). Given the singlet state lifetime, the radiative emission constant, kF, can be determined using equation 7:
![]() (7)
(7)
and is found to be kF = 3.1•107 s-1. TPrPo and TPP show radiative emission constants in the same range (kF = 3.9•107 s-1 and kF = 1.0•107 s-1, respectively). Since kF for TPPo is comparable to that of TPrPo, the sharp decrease in fluorescence cannot be attributed to this factor. In fact, a lower value of inter-system crossing for TPrPo is responsible for the enhanced luminescence of this compound (vide infra).
Triplet state properties
Triplet energy. Near-infrared emission spectroscopy was used to determine the triplet state energy, ET, of TPPo. 1-Iodopropane was chosen as a solvent to take advantage of the heavy-atom effect. By decreasing the fluorescence, the phosphorescence emission can be more easily monitored. Samples were excited with polychromatic light in the visible range to avoid homolytic cleavage of the I-C bond in the solvent. Figure 2 shows the emission spectra in air-saturated and degassed TPPo samples. In the first case, the singlet oxygen emission at 1270 nm is dominant. Upon degassing, quenching of the triplet state by oxygen is avoided and phosphorescence occurs. The difference spectrum was calculated in order to eliminate the fluorescence tail. The emission spectrum yields an estimate of the value of ET, since the real value can be anywhere between the beginning of the rise of the phosphorescence band and the peak of the same band. For consistency with the singlet state energy value, ET was calculated at 90% of the rise of the phosphorescence band, yielding a final value of ET = 124±2 kJ•mol-1 (l = 970 nm). Similar values are found for TPrPo (ET = 124 kJ•mol-1) (16), however, TPP has a much higher triplet energy (ET = 138 kJ•mol-1) (21). This value contrasts with the singlet state energies, which were very similar for all three compounds. Therefore, it can be affirmed that porphycenes present a much narrower singlet-triplet energy gap than porphyrines.
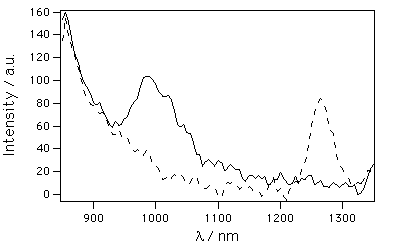
Figure 2. Phosphorescence spectrum, (---) air-saturated (–) degassed, of TPPo in 1-iodopropane.
Triplet quantum yield. Laser-induced optoacoustic calorimetry (LIOAC) was used to calculate the product fTET. Given the value of ET by near-IR emission spectroscopy, the triplet quantum yield can then be deduced. Experiments were carried out in three solvents: toluene, methylene chloride and acetonitrile. The comparison of the slopes of the energy dependence plots of the acoustic signal amplitudes obtained for TPPo and 2-BHP allowed the determination of the a value (Figure 3). Equation 4 can be rearranged to the following form:
![]() (8)
(8)
where the following values were taken: lP = 970 nm, lexc = 337 nm, lF = 667 nm and fF = 0.15. Results are summarized in Table 2.

Figure 3. Optoacoustic signal .vs. absorptivity for TPPo (o) and 2-HBP (°) in toluene
The values obtained for TPPo in acetonitrile are not included due to its low solubility, which lead to unreliable results. Values for lP, lF and fF in toluene were assumed to be similar to those in methylene chloride and were used for the experiments in this solvent. The calculated triplet quantum yield is in agreement with that of TPrPo, fT = 0.4±0.1 (9). TPP shows a much larger value (fT = 0.82) (14), like the vast majority of porphyrins.
Table 2. Results from the LIOAC experiments.
|
a |
f TET / kJ•mol-1 |
f T |
||||
|
Toluene |
0.81±0.08 |
40.9±5 |
0.33±0.04 |
|||
|
Methylene chloride |
0.83±0.03 |
34.7±2 |
0.28±0.01 |
The determination of fT allows us to calculate other kinetic variables that are important to our study. The internal conversion quantum yield, fic, can be obtained through the following equation:
![]() (9)
(9)
Using fT = 0.33 and fF = 0.15, a value of fic = 0.52 in toluene is obtained. Performing the same calculation for TPrPo and TPP leads to the surprising result of fic = 0.22 and fic = 0.05 respectively. The high value of internal conversion quantum yield is then the responsible for its low fluorescence respect to TPrPo, and its lower rate of triplet formation respect to TPP. Given fic, it is possible to calculate the value of non-radiative decay constant rate, following the equation:
![]() (10)
(10)
using tS = 4.8 ns, kic = 1.1•108 s-1 in toluene. To have a complete picture of the deactivation pathways of the singlet state, only the constant rate of inter-system crossing needs to be calculated. This can be accomplished by using the following relationship
![]() (11)
(11)
Table 3. Decay rate constants of singlet deactivation.
|
kF / s-1 |
kic / s-1 |
kisc / s-1 |
||||
|
TPPo |
3.1•107 |
1.1•108 |
6.8•107 |
|||
|
TPrPo |
3.9•107 |
2.2•107 |
4.1•107 |
|||
|
TPP |
1.0•107 |
3.8•106 |
6.3•107 |
Comparative results of the three rate constants for the three compounds subjected to study are shown in Table 3. Several features are prominent: similar values of kF are found for TPrPo and TPPo, and they are three times larger than that of TPP. This fact is consistent with the higher extinction coefficient for the porphycenes. Inter-system crossing rates are amazingly similar. However, the internal conversion rate for TPPo is almost one order of magnitude higher than the rate for TPrPo and almost two orders of magnitude higher than that of TPP. This accounts for the simultaneously reduction of fF and fT
Triplet-triplet absorption spectrum and triplet state lifetime. Laser flash photolysis (lexc = 532 nm) of TPPo in toluene resulted in a single transient species, the absorption spectrum of which is presented in Fig. 4. This species was assigned to the triplet state on the basis of oxygen quenching and efficient energy transfer from MPDME. In oxygen-free toluene solutions, the lifetime of the triplet state was tT = 51±2 ms, while in air-saturation conditions was tT = 0.21±0.01 ms. A very rough estimate of the quenching rate of the triplet by oxygen can be obtained with these two values using the Stern-Volmer equation, kq = 2.9•109 M-1s-1. For the determination of the triplet spectrum, the triplet-triplet absorption spectrum must be corrected for ground-state absorption. Therefore, an accurate value of DeT-S is needed. Three different methods were used: Comparative technique, energy transfer and complete conversion. Unfortunately, the latter two methods yielded inconsistent results. As for the comparative technique (22), flash-photolysis experiments of TPPo versus benzophenone and MPDME yielded similar values of DeT-S. (DeT-S = 11800 M-1cm-1 vs. BP, DeT-S = 12100 M-1cm-1 vs. MPDME). The final value is taken as the average value, DeT-S = 11950 M-1cm-1 at l = 420 nm in toluene. The absolute triplet spectrum is shown in Figure 4. TPrPo shows a similar spectrum, even thought the peak at 420 nm is notably sharper (9).

Figure 4. Difference T-S (o), singlet (–), and triplet (m) spectra of TPPo in toluene.
Singlet oxygen formation
Optically matched (A = 0.05 - 0.3) solutions of TPPo and a reference were irradiated with 337 nm pulses, and the luminescence decay at 1270 nm of the singlet oxygen species, 1O2, monitored. The energy dependence plots of the initial phosphorescence intensity (Io) are directly proportional to fD, and the comparison of the slopes for TPPo and the reference yields fD (Fig. 5). Perinaphthenone (PN) was chosen as the reference because its good solubility and very high singlet oxygen quantum yield in a variety of solvents. Results are summarized in Table 4.
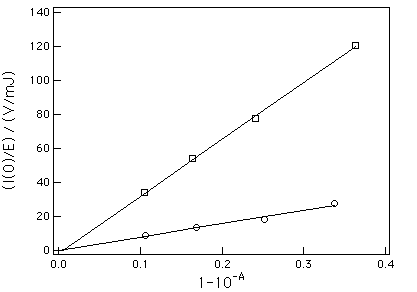
Figure 5. Slopes of the several regressions I(0)/El .vs. Absorptivity for TPPo (m) and PN (o) in toluene
Table 4. Values of singlet oxygen quantum yield
|
f D |
||
|
Benzene |
0.23±0.02a |
|
|
Toluene |
0.23±0.02a |
|
|
Methylene chloride |
0.21±0.01b |
|
|
Acetonitrile |
0.18±0.01b |
a
fD,ref = 0.93, b fD,ref = 0.97The fD values are slightly lower than those of other porphycenes (fD(Po) = 0.34±0.05, fD(TPrPo) = 0.36±0.03 in benzene) (14). Singlet oxygen production of TPP is much higher, fD = 0.64, mainly due to the high rate of triplet formation (21). SD (= fD / fT) can be evaluated, SD = 0.70±0.15, lower than that of TPrPo (SD = 1.1±0.3) and porphyrines (TPP: SD = 0.78) (21). By comparison of the 1O2 lifetime sensitized by PN or TPPo, an estimate of the quenching rate of singlet oxygen by the ground state of TPPo (assuming a much lower quenching rate of 1O2 by PN) can be calculated:
![]() (12)
(12)
using the values: tD0 = 90 ms, tD = 84 ms, and [TPPo]max = 2•10-5 M, a constant rate kq < 4•107 M-1s-1 is obtained, essentially identical to those for porphyrines (TPP: kq < 4.4•107 M-1s-1) (23).
Electrochemical studies
Redox potentials. Cyclic voltammetry was used to determine the reduction and oxidation potentials of TPPo. In methylene chloride, two near-reversible peaks were found upon reduction of TPPo. Samples in DMF also show two near-reversible peaks, but at lower potential, being TPPo more easily reduced in this solvent. All peaks were diffusion controlled, and the proposed mechanism (E) can be summarized using the following equations:
![]()
In contrast to the reduction zone, the oxidation zone presents different results depending on the solvent used. In methylene chloride, only an irreversible peak can be found, as indicated by the difference between the anodic and the cathodic peaks (ca. 0.15 V). The height of the cathodic peak was found to increase upon increasing sweep speeds, and the ratio Ip/n1/2 was not constant, which indicates that the peak is not diffusion controlled. An EC mechanism is then proposed, summarized using the following equations:
![]()
This effect is more dramatic in DMF, where the cathodic peaks completely disappear. An EC mechanism is proposed, but the chemical reaction is much faster than in methylene chloride. TPPo is slightly more difficult to oxidize than Po, TPrPo and TPP (24-26) but easier to reduce than TPrPo and TPP.
Excited states redox potentials. Before calculating the redox potential of the excited states, it was checked that the energy of the singlet state calculated using the difference between the first reduction and oxidation potentials, was consistent with the value obtained using UV-Vis spectroscopy. Indeed, a value of DE1/2 = 1.81 V in methylene chloride was obtained, which compares very well to the E00 = 1.87 eV calculated from the UV-Vis spectrum. For the singlet state, oxidation potential is found to be -0.79 V, and for the triplet (using ET instead of E00), is found to be +0.20 V. Analogously, reduction potentials are +1.14 V for the singlet and +0.55 V for the triplet.
CONCLUSIONS
The photophysical properties of TPPo obtained in this study are summarized in Table 5. TPPo shares many photophysical properties with other porphycenes (4). The introduction of the phenyl rings shifts the Q bands to the red, as expected. However, this shift is accompanied by major photochemical changes. Specifically, internal conversion is greatly increased, which results in a net loss of fluorescence and triplet formation, which in turn results in a decreased singlet oxygen quantum yield. This happens even considering that the rate constants for radiative decay and inter-system crossing remain the same when compared to the porphycene's unperturbed 18¼ aromatic system. Low level calculations suggest that even though the porphycene ring remains planar, the phenyl groups adopt a 45° angle with the plane, resulting in loss of aromaticity. Overall, even though the photochemical characteristics of TPPo are not as suitable to PDT as other sensitizers (27), recent experiments in vitro on HeLa cells (6) and a human carcinoma cell line (7) show promising features towards its further use in photodynamic therapy.
Table 5: Summary of the main properties of TPPo in organic solvents
|
Data |
Values in toluene (methylene chloride) |
|
l of the S0-S1 absorption |
659 (656) nm |
|
Molar absorption coefficient at l: e |
50000 (41000) M-1cm-1 |
|
Singlet state energy: ES |
181±2 kJ•mol-1 |
|
Fluorescence quantum yield: fF |
0.15±0.03 |
|
Singlet state lifetimea: tS |
4.8 ns |
|
Triplet state energyb: ET |
124±2 kJ•mol-1 |
|
Inter-system crossing quantum yield: fisc |
0.33 (0.27) |
|
Internal conversion quantum yield: fic |
0.52 |
|
T-S difference absorption coefficient: DeT-S |
11950 M-1cm-1 at 420 nm |
|
Singlet oxygen quantum yield: fD |
0.23 (0.21) |
|
Quenching rate of 1O2 by TPPo |
< 4•107 M-1s-1 |
|
Redox potentials |
(+1.08 V, -0.73 V, -0.98 V) |
a
In argon-saturated conditions. b In 1-iodopropane.Acknowledgments. We wish to thank Dr. J. I. Bourdelande (UAB) for assistance in the flash photolysis experiments and his personal interest in this project, Dr. E. Brillas (UB) for unvaluable help in the CV experiments, Dr. Orellana (UAM) for the SCP experiments, Dr. I. Borrell, Prof. L. Victori (IQS) and Prof. S. E. Braslavsky (MPI). F. P. especially thanks Dr. J. Abellà, C. Martí and O. Jürgens.
References
1. Vogel, E., M. Köcher, H. Schmickler and J. Lex (1986) Porphycene - a Novel Porphin Isomer. Angew. Chem. Int. Ed. Engl. 25, 257-9.2. Vogel, E., M. Balci, K. Pramod, P. Koch, J. Lex and O. Ermer (1987) 2,7,12,17-Tetrapropylporphycene - Counterpart of Octaethylporphyrin in the Porphycene Series. Angew. Chem. Int. Ed. Engl. 26, 928-931.
3. Vogel, E., M. Kocher, J. Lex and O. Ermer (1989) Steric Modulation of the Porphycene Sytem by Alkyl Substituents - 9,10,19,20-Tetraalkylporphycenes. Isr. J. Chem. 29, 257-266.
4. Braslavsky, S. E., M. Müller, D. O. Mártire, S. Pörting, S. G. Bertolotti, S. Chakravorti, G. Koç-Weier, B. Knipp and K. Schaffner (1997) Photophysical Properties of Porphycene Derivatives (18¼ porphyrinoids). J. Photochem. Photobiol. B 40, 191-198.
5. Nonell, S., N. Bou, J. I. Borrell, J. Teixido, A. Villanueva, A. Juarranz and M. Cañete (1995) Synthesis of 2,7,12,17-Tetraphenylporphycene (TPPo) - First Aryl-substituted Porphycene for the Photodynamic Therapy of Tumors. Tet. Lett. 36, 3405-3408.
6. Cañete, M., M. Lapena, A. Juarranz, V. Vendrell, J. I. Borrell, J. Teixidó, S. Nonell and A. Villanueva (1997) Uptake of Tetraphenylporphycene and its Photoeffects on Actin and Cytokeratin Elements of HeLa Cells. Anti-Cancer Drug Design 12, 543-554.
7. Villanueva, A., M. Cañete, S. Nonell, J. I. Borrell, J. Teixidó and A. Juarranz (1996) Photodamaging Effects of Tetraphenylporphycene in a Human Carcinoma Cell Line. Anti-Cancer Drug Design 11, 89-99.
8. Magde, D., J. H. Brannon, T. L. Cremers and J. Olmsted (1979) Absolute luminescence yield of cresyl violet. A standard for the red. J. Phys. Chem. 83, 696-699.
9. Nonell, S., P. F. Aramendia, K. Heihoff, R. M. Negri and S. E. Braslavsky (1990) Laser-Induced Optoacoustics Combined with near IR emission - An Alternative Approach for the Determination of Intersystem crossing Quantum Yields Applied to Porphycenes. J. Phys. Chem. 94, 5879-5883.
10. Compton, R. H., K. T. V. Grattan and T. J. Morrow (1980) Extinction Coefficients and Quantum Yields for Triplet-triplet Absorption Using Laser Flash Photolysis. J. Photochem. 14, 61-6.
11. Bonnet, R., A. A. Charalambides, E. J. Land, R. S. Sinclair, D. Tait and T. G. Truscott (1980) Tripet States of Porphyrin Esters. J. Chem. Soc. Faraday Trans. 1 76, 852-9.
12. Moore, T. A., D. Benin and T. Roderick (1982) Photoacoustic Mesurement of Photophysical Properties. Lowest Triplet State Energy of a Free Base Porphyrin. J. Am. Chem. Soc. 104, 7356-7.
13. Oliveros, E., P. Suardi-Muraseco, T. Aminian-Saghafi, A. M. Braun and H. J. Hansen (1991) 1H-Phenalen-1-one - Photophysical Properties and Singlet Oxygen Production. Helv. Chim. Acta 74, 79-90.
14. Nonell, S., Ph. D. Thesis, Max Planck Institut für Stralenchemie (Mülheim a. d. Ruhr).
15. Dorough, G. D., J. R. Miller and F. M. Huennekens (1951) Spectra of the Metallo-derivatives of a,b,g,d-tetraphenylporphyne. J. Am. Chem. Soc. 73, 4315-20.
16. Aramendia, P. F., R. W. Redmond, S. Nonell, W. Schuster, S. E. Braslavsky, K. Schaffner and E. Vogel (1986) The Photophysical Properties of Porphycenes: Potential Photodynamic Therapy Agents. Photochem. Photobiol. 44, 555-559.
17. The Porphyrins Vol. III, Ed D. Dolphin Academic Press Inc. (1978), 36.
18. Harriman, A. (1980) Luminescence of Porphyrins and Metalloporphyrins. J. Chem. Soc. Faraday Trans. I 76, 1978-85.
19. IUPAC Solubility Data Series Vol. 7 (Pergamon), 257. 7, p.257.
20. "Handbook of Organic Photochemistry", CRC Press Inc. Boca Raton, Fl. (1989), Vol I, Ch. 8, 231-237,327-355.
21. Darwent, J. R., P. Douglas, A. Harriman, G. Porter and M. C. Richoux (1982) Metal Phthalocyanines and Porphyrines as Photosensitizers for Reduction of Water to Hydrogen. Coord. Chem. Rev. 44, 83-126.
22. Bensasson, R. V., C. R. Goldsmith, E. J. Land and T. G. Truscott (1978) Laser Intensity and the Comparative Method for Determination of Triplet Quantum Yields. Photochem. Photobiol. 28, 277-281.
23. Tanelian, C. and C. Wolff (1988) Mechanism of Physical Quenching of Singlet Molecular Oxygen by Chlorophylls and Related Compounds of Biological Interest. Photochem. Photobiol. 78, 277-280.
24. In The Porphyrins Vol. V, Ed D. Dolphin Academic Press Inc. (1978), 143 (Edited by pp.
25. Gisselbrecht, J. P., M. Gross, M. Köcher, M. Lausmann and E. Vogel (1990) Redox Properties of Porphycenes and Metalloporphycenes as Compared with Porphyrins. J. Am. Chem. Soc. 112, 8618-8620.
26. Renner, M. W., A. Forman, W. Fu, C. K. Chang and J. Fajer (1989) Electrochemical, Theoretical and ESR Characterizations of Porphycenes - The ¼ Anion Radical of Nickel (II) Porphycene. J. Am. Chem. Soc. 111, 8618-8621.
27. Aveline, B., T. Hasan and R. W. Redmond (1994) Photophysical and Photosensitizing Properties of Benzoporphyrin Derivative Monoacid Ring A (BPD-MA). Photochem. Photobiol. 59, 328-335.