Experiment: Diels-Alder Reaction
Week 5
- How many carbon signals would you expect to see in the starting materials (cyclopentadiene, maleic anhydride) of this week's experiment? Sketch rough spectra of each compound.
- How many carbon signals would you expect to see in the dimerization product of cyclopentadiene?
- Diels-Alder components react more easily if one reaction partner is electron-rich and the other is electron-poor.
This combination is the most common type of pairing. There are also cases where the electron distribution is reversed (inverse electron demand).
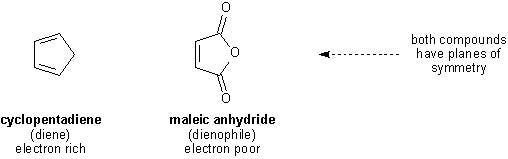
This week's Diels-Alder reaction is an extremely easy one to perform because it involves an electron-rich diene and extremely electron poor dienophile.
Furthermore, both diene and dienophile have planes of symmetry, thus allowing only two possible orientations (exo and endo) before the cycloaddition occurs.
- Suppose you had the following Diels-Alder reaction partners:

What products would you expect from this reaction? Which one would be the major product using the same reaction conditions as in this week's experiment?
What do you think the carbon and proton NMR's of the products of this reaction would look like?
- From scratch, generate the energy orbital diagrams of maleic anhydride and determine which level is the HOMO and which is the LUMO. (Don't peek at the reader!)
- Have you noticed a theme in this week's, "Think About It"?