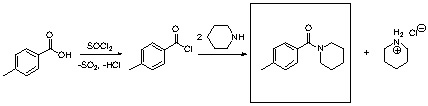
1. a. The first step of the reaction yields an acid chloride, while the second one affords an amide. Note that two equivalents of piperidine are needed for the reaction: one molecule ends up in the amide, the other one to quench the HCl side product.

b. If the student does not convert the p-toluic acid into the acid chloride, he would observe an acid-base reaction that yields a piperidinium salt. This salt can be converted into the amide by heating it to ~200 oC, which is not the case in this reaction.
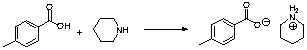
c.
Reaction 1:
p-toluic acid: m.w.=136 g/mol
nA = 6.8 g/136 g/mol = 0.050 mol (= limiting reagent)
Thionyl chloride: m.w.=119 g/mol, d=1.631 g/cm3
nT = 5.0 mL * 1.631 g/mL / 119 g/mol = 0.069 mol
p-toluic chloride: m.w.=154.5 g/mol, yield = 90%
nC = 0.050 mol * 0.90 = 0.045 mol (=6.95 g)
Reaction 2:
p-toluic chloride: 0.045 mol (=limiting reagent)
Piperidine: m.w.=85 g/mol, d=0.862 g/cm3
nP = 10.0 mL * 0.862 g/mL/85 g/mol = 0.10 mol
Product: m.w.= 203 g/mol, yield = 80%
nTP = 0.045 mol * 0.80 = 0.036 mol
weight = 0.036 mol * 203 g/mol = 7.31 g of amide
1.d.

Notes: After both reactions, the reaction mixture contains the amide, the unreacted acid and acid chloride and piperidine. Piperidine (a base) can be removed by extraction with dilute hydrochloric acid, while the acid and some of the acid chloride are removed by extraction using a dilute base. The remaining acid chloride and the amide are separated by column chromatography using a polar stationary phase and a non-polar solvent.
e. The reactant A has a carbonyl stretching mode at ~1700 cm-1 and a broad peak from 2500-3500 cm-1 that is due to hydrogen bonded OH groups. The product shows a shift to 1640 cm-1 for the carbonyl function (due to the amide resonance). There will not be a peak in the 3300-3500 cm-1 range because the product is a tertiary amide.
2.a. The reflux has two reasons: first of all, to dissolve more of the ligand, which does not dissolve well in cold ethanol, and also to increase the rate of the reaction.
b. The oxygen in air is used as oxidant in this reaction.
c. Heptane is added to lower the polarity of the solution, which means that polar catalyst precipitates out, while the unreacted ligand, which is less polar remains in solution. If the dichloromethane were boiled off, both of them would precipitate together.
d. The coordination geometry around the Mn atom is best described as a square pyramid. The two oxygen atoms and the two nitrogen atoms of the ligand form the basal plane, while the chlorine atom occupies the apex. The Mn atom sits in the center of the pyramid.

3.a. The rotary evaporator is used to evaporate solvents. It does this usually faster than doing the same step on the hotplate because the spinning motion increases the surface area. Since it can also be done under reduced pressure, this technique is also more compatible with compounds that tend to decompose easily at higher temperatures.
b. Sodium bicarbonate is usually used to remove acids from a mixture. In those cases, carbon dioxide is formed as a byproduct. Thus, the pressure in the separatory funnel increases and it has to be vented more frequently to prevent any accidents to happen.
NaHCO3 + H+ ----- > Na+ + H2O + CO2
c. Deuterated solvents are used to allow the NMR instrument to lock the magnetic field. The NMR instrument looks at the deuterium resonance to do so.
d. The advantage of a smaller mesh size is that there is more surface area that is active for the separation of compounds. A disadvantage is that the column packs tighter, which means that normally flash chromatography has to be used to increase the rate of elution.
e. The optimal pH-value is between 9.5 and 11.5 for the epoxidation reaction. This is accomplished by adding the phosphate buffer. If the pH value is too low, the alkene is going to be chlorinated. At higher pH-values, the catalyst decomposes and the epoxide is also hydrolyzed. The wavelength and the molar absorptivity of the peak should be reported.
f. Most chalcones are relatively non-polar. Thus, a non-polar solvent should be used e.g. hexane, dichloromethane, etc. Acetone would be a poor choice since it potentially overlaps with the peak at l=320 nm. Since the UV range is of interest here, either a silica cuvette or a quartz cuvette should be used here. Plastic cuvettes would dissolve in the solvents above as well.
g. THF contains peroxides, water and oxygen. These impurities can be removed by refluxing the solvent over a mixture of sodium and benzophenone. The solvent is dry and clear of peroxides if the color changes to a dark-blue (formation of the ketyl radical!).
2 Na + 2 H2O ---- > 2 NaOH + H2
4. a.

Note that there are three equivalents of stannous chloride required and seven equivalents of hydrochloric acid, which means that the solution has to be strongly acidic.
b. Glacial acetic acid is used as solvent for the nitro compound, because it is not polar enough to dissolve in the concentrated stannous chloride solution. In addition, it also provides additional protons to form the ammonium salt that precipitates from the solution.
c. The potassium carbonate was used as drying agent for the amine solution. K2CO3 is basic and therefore does not react as strongly with the amine itself. Sodium sulfate and magnesium sulfate also form adducts with bases, which would cause a loss of product.
d. The two peaks of the nitro group (~1300-1400 and ~1500-1600 cm-1) will disappear, while two peaks in the range from 3300-3500 cm-1 and a broad peak at 1600 cm-1 will appear characteristic for the primary amine function.
5.a.
 |
Since the stationary phase is polar and the mobile phase is non-polar, only non-polar compounds like ferrocene will move significantly here. Diacetylferrocene is the most polar compound in this mixture, thus basically remaining on the starting line, while the target compound, acetyl ferrocene, will move a little higher up the plate. |
5.b.
 |
The stationary phase is still polar, but the addition of dichloromethane increased the polarity of the mobile phase. Ferrocene will basically move with the solvent front, while the acetylferrocene and diacetyl-ferrocene will now higher up the plate as well. |
6.a.

A mixture of concentrated nitric and sulfuric acid is used to generate the nitronium ion in this reaction.
b. Sulfuric acid has two functions here. It dissolves the ester and makes the reaction therefore homogeneous and secondly also serves as acid for the formation of the nitronium ion (see above).
c. The nitration reaction itself is highly exothermic reaction. The nitronium ion is a very strong electrophile, which easily can lead to polynitration. Unfortuantely, the activation energy for a dinitration is not so much higher than the one for the mono-nitration. Thus, proper temperature control during the reaction minimizes the amount of dinitration product formed in the reaction. This is accomplished by placing the reaction mixture in an ice bath, and adding the acid mixture slowly.
d. The meta isomer is formed here because the ester group (-COOMe) is an electron-withdrawing group. The system tried to avoid the accumulation of positive charges on the same carbon atom, what would happen if the substitution occurs in ortho or para position.
e. The nitronium is a very strong electrophile since it has only one resonance structure that follows the octet rule. The positive charge is located on the nitrogen atom there. In case of the acylium ion there are two resonance structures, of which the one with the positive charge is located on the carbonyl carbon atom. There is very little of the electrophile present in solution, which make it a poor electrophile overall. The nitronium ion is able to nitrate electro-poor arenes, while the acylium ion only reacts with electron-rich systems e.g. toluene, ferrocene, etc.
7.a. The stationary phase is chiral and consists of b-cyclodextrin. The separation is based on the different interaction of the enantiomers with the chiral stationary phase.
b. In order to determine the e.e. values, first the compounds in the spectrum have to be assigned. Compound B and compound C have very similar retention times, which means that they are most likely due to the cis and the trans epoxide. Compound A is most likely due to the unreacted alkene, since it has a much shorter retention time.
Compound B: AB = 0.5*(5.5 mm *34.5 mm) = 95 mm2
Compound C: AC = 0.5*(9 mm * 12.5 mm) = 56 mm2
e.e.= (AB-AC)/(AB+AC) * 100 % = (95-56)/(95+56) * 100% = 26%
c. The sample is dissolved in a low boiling solvent like diethyl ether or dichloromethane. The concentration should not exceed 5 mg/mL of solvent. Generally, about 1mL is injected into the GC instrument in order to avoid overloading of the column, which would lead to poor separation of the compounds.
8. a. Since ibuprofen is a chiral carboxylic acid, a chiral base is needed to separate the two enantiomers from each other. a-Phenylethylamine would be a good choice for this separation. The resulting ammonium salts are diastereomeric and therefore possess different solubilities in water.
b. Since the optical rotation of the sample is negative, the majority of the sample has to be the (R)-form.
optical purity = (observed optical rotation)/(optical rotation of the pure enantiomer)
= -23.2o/(-58o) * 100 %
= 40%
This means that the mixture contains 70% of the R-enantiomer and 30% of the S-enantiomer. Thus the maximum yield of the (S) form is 7.5 g (=25g*0.30).
c. The optical purity can be determined using a chiral shift reagent or using a chiral stationary phase in a GC or HLPC run.
d. Extra Credit: Advil and Motrin
9. a. Alkyl bromides are cheaper than alkyl iodides and much more reactive than alkyl fluorides.
b. The Grignard reagent formed CH3OCH2CH2MgBr has a good leaving group (OCH3), which means that the system will eliminate readily yielding ethylene and MgBr(OCH3).
c. A good solvent is able to dissolve the compound, but does not react with it. In case of the dichloromethane the protons are more acidic than the ones on most Grignard reagents, which means that they are removed by a strong base. Dimethylsulfoxide possesses an electrophilic sulfur atom, which react with the nucleophilic Grignard reagent. Ethers do not possess acidic proton or electrophilic centers, and therefore react less readily with Grignard reagent. In addition, they are polar enough to dissolve most Grignard reagents.
d. Oligomers are species that have three or more units combined, but are not extreme in terms of association. The molecularity can be reduced by using a more polar solvent or by adding compounds like TMEDA, which is able to complex the Mg-ion better.
10. a. The degree of unsaturation is given by
D.B.= (7*2+2-12)/2 = 2
b. The IR spectrum shows peaks at 3000-3040 (CH, sp2), 2850-2980 (CH, sp3), 1675 (C=O, conjugated), 1615 (C=C, alkene), 1450, 1370 (CH2, CH3, bend), 970 cm-1 (oop, trans alkene)
c. The signal at d=0.85 ppm (t, 3H) is due to a methyl group next to a methylene group. The multiplet at d=1.5 ppm (m, 2H) indicates the presence of a longer chain (n>2). The two signals overlapping at d=2.3 ppm (s, 3H and t, 2H) are a result of an isolated methyl group and a methylene group next to a CH2 group. The doublet at d=6.1 ppm (d, 1H, J=15 Hz) is due to an alkene proton that is in trans position to another alkene proton. Finally, the signal at d=6.8 ppm (dt, 1H, J=15 Hz) is due to the second alkene proton that has an additional CH2 function adjacent.
d. There are seven signals in the 13C-NMR spectrum for seven carbons in the empirical formula, which means that there is no symmetry in the compound. The signal at d=197ppm is due to a ketone (quartenary carbon). The signals at 131 and 148 ppm are CH groups that belong to an alkene. The range between 13 and 35 ppm shows two CH2 and two CH3 groups.
e. Based on the discussion above the molecule is trans-3-Hepten-2-one.
![]()