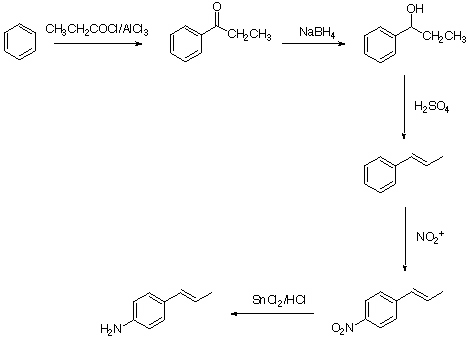
2. The esterification is an equilibrium reaction, where water is one of the products. The presence of water (usually in the PhCOOH due to insufficient drying) causes a lower yield of the ester. Show equation.
3. The presence of water lowers the concentration of the nitronium ion. The reaction slows down. Show equation.
b.
1. To convert PhCOOMgBr (after carbonation) to PhCOOH. Also to dissolve unreacted Mg-turnings.
2. It serves as a catalyst to protonate the carbonyl oxygen on the acid and make it a better electrophil for the weak nucleophil methanol.
3. As solvent for the ester (PhC(OH)OMe+) and to generate the nitronium ion.
3.
a. The NMR spectrometer needs a D-atom as a reference for the lock.
b. It is due to CDCl3. (for D: I=1, 2xI+1=3)
c. The spectrum is proton decoupled which increases the sensitivity and simplifies the spectra. The coupling information can be obtained from the DEPT spectrum.
4.
a. TLC is a micro technique. Small spots with small quantities are required in order to get good separation and avoid tailing.
b. The polarity of the compound in regard to the stationary phase is the important criterium here. Therefore, the naphthalene has the highest Rf value (non-polar), then the amide, the alcohol and finally the acid (most polar) with the lowest Rf value.
c. A UV lamp was used (fluoresence indicator on TLC plate)
5.
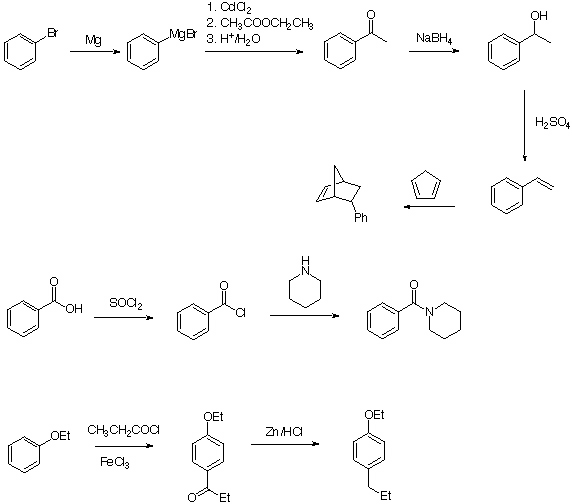
6.
Pathway 1 is the preferred way. The reaction of the acid with thionyl chloride leads to the acid chloride which is more reaction that the acid itself. Chloride is a much better leaving group than the hydroxy group. The reaction actually proceeds at room temperature within a reasonable amount of time (Schotten Baumann Esterification).
The problem with pathway 2 is that the tertiary alcohol will eliminate water very easily to afford butene, and not (or very little ester). Remember that the elimation for tert. alcohols can be done under much milder conditions than for prim. or sec. alcohols.
7.
a. The compound contains one Cl-atom (see M/M+2 ratio ~ 3:1). One of the main fragments that is cleaved off is m/z=43, which indicates the presence of an acetyl group. Knowing this and the molecular weight, the formula is C8H7OCl (p-chloroacetophenone).
b. m/z=135 is due to the acylium ion (ClC6C4CO+) and m/z=111 is ClC6H4+
8. IR spectrum
| wavenumber | assignment |
| 2800-2900 | sp3 CH |
| 1700 | C=O |
| 1600, 1500 | C=C (aromatic) |
| 1250, 1040 | C-O-C (ester) |
Proton NMR
| Shift | Assignment |
| 6.5-7.0 (2d, 4H) | para subst. ring |
| 3.6 (s, 3H) | OCH3 |
| 2.6 ('t', 4H) | X-CH2-CH2-Y |
| 2.0 (s, 3H) | X-CH3 |
DEPT spectrum
| Shift | Assignment |
| 208 | C=O (quartenary) |
| 158, 132 | aromatic C (quartenary) |
| 130, 114 | aromatic CH |
| 55 | OCH3 |
| 45 | CH2 |
| 30 | CH3 |
| 29 | CH2 |
Answer:
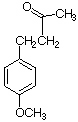
9. There are several acceptable ways and reagents to obtain this compound. Here is one example.