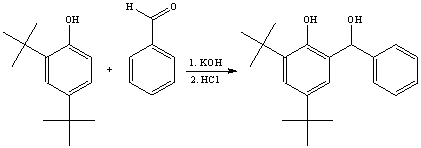
1. a. The reaction shown is similar to the Lederer-Mannase reaction carried out in the lab. The phenol is deprotonated and the resulting phenolate then acts as a nucleophile.

The resulting product is a secondary alcohol in this case, because an aldehyde other than formaldehyde was used.
b. The solvent has to be fairly polar in order to dissolve the reactants (A, B and KOH) and the intermediates well, and should not react with the reactants or products. As such, methanol or ethanol would be good choices here.
c. Compound (B) should be used in excess because of two reasons. First, benzaldehyde in a liquid, while the product is expected to be a solid (m.p.=91-93 oC) due its high molecular weight and its polarity. Even though compound (A) has a lower polarity than the product, it still is a solid and it will be more difficult to separate them by recrystallization.
d. If acetophenone is used instead of benzaldehyde, complications arise due to the formation of enolate ions that cause the formation of aldol condensation products as shown below.
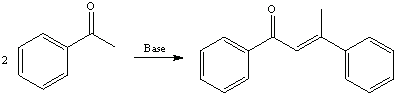
The phenolate can act as a nucleophile and as a base, which deprotonates the ketone instead of adding to the carbonyl group. Due to this side reaction, the yield for product (P) is decreased.
e.

1 2 3 |
The stationary phase (Al2O3, alumina) is polar and the mobile phase is weakly polar. Thus, more polar compounds interact stronger with the stationary phase than non-polar compounds. As a result, the product (P) has the lowest Rf-value while the aldehyde (B) has the highest Rf-value. |
f. In order to the yield, first the limiting reagent must be identified.
Moles of A: nA= 3.71 g/(206.32 g/mol) = 0.0180 mol
Moles of B: nB= 2.0 mL * 1.045 g/mL/(106.12 g/mol) = 0.0197 mol
Moles of KOH: nKOH= 2.00 g/(56.1 g/mol) = 0.036 mol
Thus, compound A is the limiting reagent. The experimenter expects to obtain 0.018 mol of the product as well.
Moles of P: nP=4.78 g/(312.45 g/mol) = 0.0153 mol
Thus, the yield for the reaction is given by
Yield = nA/nP * 100% = 85%
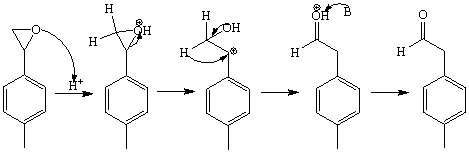
2. a. The retention time for compound A is tR=4.1 min.
b. In order to calculate the e.e.-value, first the peaks have to be assigned properly. Most likely peak A and peak B are due to the two enantiomers of the epoxide. First the individual areas have to be determined.
Area of A: AA= (35 mm * 7.5 mm)/2 = 131.3 mm2
Area of B: AB= (11 mm * 3 mm)/2 = 16.5 mm2
Total area: AT= 147.8 mm2
e.e. = (131.3 – 16.5) / 147.8 *100% = 77.7 %
c. The third peak is due to a reaction that took place on the column. The epoxide underwent an acid-catalyzed rearrangement to form the aldehyde.
3.a. The nitronium ion is obtained from the reaction of conc. nitric acid (which acts as a base) and conc. sulfuric acid (which acts as an acid).
HNO3 + H2SO4 ---- > NO2+ + H2O + HSO4-
b. The nitronium ion is a very strong electrophile because only resonance form is reasonable here, which carries the positive charge on the nitrogen atom. The acylium ion is a weak electrophile because there are two resonance forms, and the one needed for the EAS is the minor contributor. In order to avoid polynitration, the nitration reaction has to be cooled. On the other side, the acylation reaction is very slow and the higher temperature increases the rate of the reaction significantly.
c. The Friedel-Crafts alkylation produces a product that is actually more reactive than the original reactant because the alkyl groups are weak donor ligands. Thus, here often times polyalkylation is observed. One the other hand, the Friedel-Crafts acylation only allows performing one EAS, because the product is usually significantly less reactive than the reactant. The subsequent reduction (Wolff-Kishner or Clemmensen) can be used to convert the acyl group into an alkyl function. The second procedure generally allows for more control over the degree of substitution.
d.
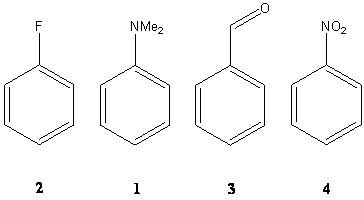
The dimethylamino group is a good donor due to its lone pair on the nitrogen atom, and therefore a strongly activating group. On the other hand side, the nitro group is a strongly deactivating group due to electropositive character of the nitrogen attached to the ring which does not have a lone pair. The fluoro ligand is weakly deactivating while the aldehyde function is moderately deactivating.
4. a. Ethers react with oxygen in air to form peroxides. In addition, they are more or less polar and absorb water readily. Both contaminants can be removed efficiently using sodium metal and benzophenone. The reaction of water with sodium is given below.
![]()
The sodium actually reacts with the benzophenone to form a ketyl radical that is dark blue in color, which will appear as soon as the contaminants are destroyed.
b. By comparison of the actual melting point with the literature melting point, which is much lower, it is clear that the student did not isolate the target compound. Since the observed melting point is only below the melting point of the byproduct, it is safe to assume that the student isolated mainly the byproduct. Since the melting point is fairly broad, it has to be contaminated with the product or the reactant.
c. The saturated sodium bicarbonate solution is usually used to remove acids from the reaction mixture. As a result, carbonic acid is formed which decomposes to form carbon dioxide and water.
![]()
The evolution of carbon dioxide will cause a pressure build-up in the extraction container i.e. centrifuge tube. This is why the procedure asks to vent the container frequently.
d. Whenever a NMR spectrum is acquired a, a deuterated solvent should be used because the NMR spectrometer requires a reference point to set up the proper range to be measured (“lock”). Deuterochloroform is used because it is reasonably priced and has the ability to dissolve a broad variety of organic compounds as well.
e. The peak at =2350 cm-1 (usually a doublet) is due to carbon dioxide in air. It represents the asymmetric stretching mode for the CO2 molecule. The relative size changes because of the sample concentration and the quality of background correction.
f. The experimenter can draw two conclusions from this pattern. First, the molecule contains two chlorine atoms, which should be evident from the cluster pattern (M:M+2:M+4=100:65:10). Secondly, the molecule contains nine carbon atoms (M:M+1=~9).
g. The carbonyl stretching frequency in the chalcone is fairly low because of a strong conjugation of the C=O function with the ferrocene and the newly formed double bond.
h. The use of dichloromethane and a quartz cuvette allows the experimenter to observe a relative large range since neither one absorbs significantly above 250 nm. Solvents like acetone would interfere with the measurement at much higher -values. The same applies to most polymer based cuvettes.
5. a. Ethanol is used as solvent because the aldehyde and diamine should be in solution in the beginning while the ligand that is relatively non-polar precipitates from solution.
b. Water is added after the reaction is completed and while the reaction mixture is still hot. This step increases the polarity of the solution, which in turn causes more precipitate to form.
c. The intramolecular hydrogen bonding is evident in the TLC, the 1H-NMR spectrum and the IR-spectrum. Despite having two hydroxyl groups the ligand moves fairly high up the TLC plate. The shift of the phenolic proton to =~13.6 ppm is another strong piece of evidence for this hydrogen bond. Finally, the stretching mode of the OH function is shifted to much lower wavenumbers (2500-3200 cm-1).
d. In order to evaluate the optical purity, first the concentration has to be found.
c=0.150 g/10 mL= 0.015 g/mL
Next, the specific optical rotation for the sample has to be found
[]= /(c*l) = -4.7o/(0.015*1) = -313.3o
The optical purity is then given by
Optical purity = -313.3o/(-315o) * 100% = 99.5 %
This value indicates a very high optical purity that shows that the resolution step was successful.
6. a. The third step is the formation of the lidocaine in a SN1-reaction between the chloroanilide and diethylamine. Diethylammonium chloride is obtained as byproduct in this reaction.
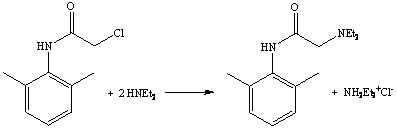
b. Since toluene is used as a solvent, which is pretty non-polar, the byproduct of the reaction precipitates from the solution as a white solid.
c. The treatment with water removes the excess diethyl amine from the solution. The subsequent extraction with hydrochloric acid transfers the lidocaine into the aqueous layer due to the protonation of the diethylamino function in the molecule. The unreacted chloroanilide remains in the organic layer.
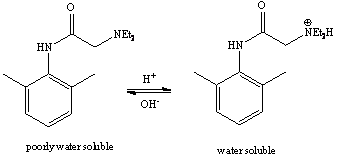
d. The addition of potassium hydroxide deprotonates the diethylammonium function, which causes the lidocaine to form, either as a yellow as oil on the top or as a white, waxy solid.
7. a. The catalyst has two main functions. First of all, it acts as an oxygen transfer reagent by being oxidized by bleach in the aqueous layer and then transferring the oxygen to the alkene in the organic layer. Secondly, it also introduces the chirality to the reaction by being chiral itself and therefore controlling the access of the alkene to the Mn=O function.
b. Bleach is used as oxygen source in this reaction. The main reason is that it is much cheaper than MPCBA or iodosobenzene. However, this also means that the reaction has to be carried out at temperatures significantly above 0 oC, which causes a slight loss is stereoselectivity for the reaction.
c. The phosphate buffer (Na2HPO4) is used to main a stable pH-value between pH=9.5-11.5. The problem is that at lower pH-values, the alkene is chlorinated, the epoxide is hydrolyzed and the catalyst is gradually destroyed as well. If the pH-value is too high, the catalyst and the epoxide are destroyed.
d. There is a broad variety of parameters that determine how fast the reaction progresses i.e. catalyst loading, pH-value, stirring, presence of promoter, etc. In order to optimize the time and the yield, TLC is used to monitor the progress. First the alkene is visualized using UV-light, and then the epoxide is visualized using ceric staining.
e. The mobile phase chosen for flash chromatography should cause a good separation of the product from the reactant and the byproducts. In addition, the product should move reasonably well in order to be able to elute it off the column at a reasonable time and a reasonable amount of solvent. This is why the Rf-values of Rf=~0.4 and Rf=~0.8 are chosen for the epoxide and the alkene, respectively.
f. The first hexane treatment is to wet the column. Then, the 1% triethylamine solution is added to neutralize the acidic residues on the column. This step reduces the acid-catalyzed rearrangement of the epoxide on the column. The second hexane treatment is used to remove the access triethylamine from the column.
g. Only the surface of the stationary phase used for the separation. Thus, a relatively large amount of material is needed to ensure the entire sample to be separated can be absorbed.
h. The Jacobsen epoxidation allows forming epoxides for unfunctionalized alkenes, which means that it has a much broader scope. The Sharpless method is limited to allylic alcohols as substrates.
8.a. The esterification is an equilibrium reaction with a low Keq-value (1-10). This means that the yield for the reaction will be relatively low. Since water is one of the products in the reaction, the yield would decrease even further.
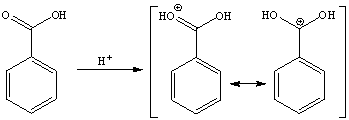
b. The carboxylic acid itself is not a particularly good electrophile. The addition of the sulfuric acid causes the carbonyl group of the carboxylic acid function to be protonated, which makes the carbon atom a better electrophile because the second resonance form (with the positive charge on the carbon atom) because more of a contributor.
c. The addition of water causes a phase separation. While most of the methanol and the sulfuric acid dissolve in the aqueous layer, the ester and the unreacted benzoic acid form the organic layer. The addition of the water has to be done after the cooling step to minimize the hydrolysis of the ester.
d. The extraction with saturated sodium bicarbonate solution is used to remove the unreacted benzoic acid and the sulfuric acid from the mixture. Carbonic acid is formed which decomposes to form carbon dioxide and water.
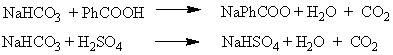
The resulting salts (NaHSO4 and NaPhCOO) are water soluble.
e. The crude product can be further purified by vacuum distillation. A normal distillation would not be a good choice here because the compound boils at Tb=~200 oC. A significant amount of product would decompose during the distillation at such high temperatures.
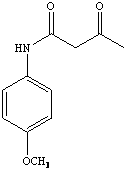
9. a. Degree of unsaturation: D.U.= (2*C+2-H+N)/2 = (2*11+2-13+1)/2 = 6
b. The most important peaks are at (in cm-1): 3251 (NH), 3004-3138 (CH, sp2), 2963 (CH, sp3), 1715 (C=O, ketone), 1666 (C=O, amide), 1515, 1608 (C=C, aromatic), 1361, 1455 (CH2, CH3, bend), 841 (oop, para-subst.)
c. The 13C-NMR spectrum shows nine peaks, which means that the target molecule has to be fairly symmetric because the molecular formula shows eleven carbon atoms.
d. The 1H-NMR spectrum shows six signals, three of them are singlets and two of them are doublets. The broad signal at d=9.0 ppm (1H) is due to the NH of an amide. The two doublets at d=6.84 ppm (2H) and 7.42 ppm (2H) are due to a parasubstituted benzene ring. The remaining singlets at d=3.77 ppm (3H), 3.54 ppm (2H) and 2.29 ppm (3H) are due to methyl or methylene groups that are attached to electron-withdrawing atoms or groups.
e. The 13C-NMR spectrum exhibits nine signals. The signals at 131, 157, 164 and 205 ppm are due to quaternary carbon atoms. The two later ones are from the carbonyl group of amide and the ketone group. The signals at 114 and 122 ppm are due to CH functions from the benzene ring. The signals at 31 and 55 ppm are a result of two methyl groups. Finally, the signal at 50 ppm is due to a methylene group.
f. Based on the discussion above, compound Z is 4-methoxyacetoacetanilide
Did you pass?