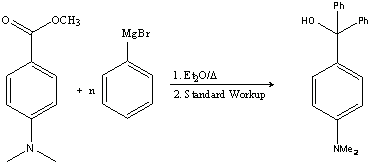
1.a. The reaction below shows the reaction of a Grignard reagent with an ester. Note that two Ph-groups are added to the ester, because the intermediate formed in the reaction (ketone) acts an electrophile as well. The final product is a tertiary alcohol.

b. Grignard reagents are good nucleophiles and strong bases. Thus, they react with electrophiles and acids. Since ethers are more or less polar, they tend to absorb moisture from the air. The water reacts with the Grignard reagent and destroys it. Hence, water should be excluded from the reaction as much as possible i.e. by using anhydrous solvents, etc.
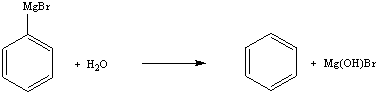
c. The addition of hydrochloric acid is necessary to quench the left over Grignard reagent and to convert the magnesium alcoholate into the alcohol.

If pH-value was too low, the dimethylamino group would be protonated as well, making the product (P) much more water soluble.
d. Compound B, the Grignard reagent can be obtained by the reaction of Mg-metal with bromobenzene in diethyl ether as a solvent.
e. In order to determine the yield, first the limiting reagent has to be found.
Moles of A: nA= 2.70 g/179.22 g/mol= 0.015 mol
Moles of B: nB= 0.020 L* 2 mol/L = 0.040 mol
Since one mole of compound A reacts with two moles of compound B, 0.030 mol of compound B would be needed to consume all of compound A. Compound B is available in larger amount, which means that compound A is the limiting reagent. Thus, 0.01275 mol of the product are produced assuming a 85% yield.
Moles of P: nP=0.015 mol * 0.85 = 0.01275 mol
Weight of P: mP = 0.01275 mol * 303.40 g/mol = 3.87 g
The reaction should yield 3.87 g of the product P.
f. Tetrahydrofuran (THF) is more polar than diethyl ether and much more miscible with water. As a result, it is much more difficult to observe a phase separation when water or hydrochloric acid is added. The miscibility also causes problems because the product is much more soluble in the solvent mixture, which makes it more difficult to extract it.
g. The amino group possesses two acidic protons that are going to react with the Grignard reagent. Thus, the majority of the Grignard reagent will be destroyed in this acid-base reaction.
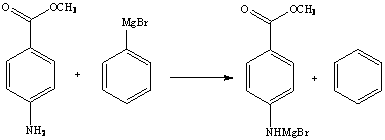
After the work-up, the starting material would be recovered since the NH function would be reprotonated.
2. a. The sodium hydroxide deprotonates the hydrochloride to produce the actual catalyst (thiamine). Without this step, the condensation would not occur at all.
![]()
b. Older batches of benzaldehyde usually contain significant amounts of benzoic acid due to the oxidation of the aldehyde function by oxygen in air. This poses a problem because the acid destroys the catalyst (see above), and the condensation reaction slows down or does not occur at all.
c. The term “homogeneous” means that all reactants are in the same phase, which is a solution here. If not mixed properly, two layers are observed in the reaction, which would slow down the reaction and also cause several side reactions to occur i.e. oxidation of benzaldehyde.
d. Occasionally the solution supersaturates in this experiment, which means that the solubility limit is exceeded but the compound does not precipitate due to other reasons. The easiest way to solve this problem is by scratching the inside walls with a glass rod. Alternatively, the solution could be seeded with a benzoin crystal.
e. He would observe that the filter paper would float in the mixture and that a lot of his product would get sucked into the filter flask because the holes in the funnel are too large to hold back the crystals. The proper protocol would be to first place the filter paper onto the plate, apply the vacuum and then pour the mixture on the filter paper.
3. a. The extractions in Chem 30BL are carried out at room temperature. Thus, the compound should exhibit a high solubility in the solvent at room temperature in order to be a good solvent for extraction. However, for recrystallization the solubility at room temperature should be fairly low in order to recover a significant amount of the product in this procedure. Since a solvent cannot fulfill both requirements, it can only be used in either technique.
b. The solvent should dissolve the reactants well, but not the product or byproduct in order to simplify the separation of the product and the monitoring of the reaction. A wide temperature range (low melting point and reasonably high boiling point) is desirable as well in order to be able to cool a mixture (to slow the reaction down) or heat it to reflux (to speed up the reaction). The solvent should not react with the reagents or the product.
c. The choice of solvent is very important in chromatography. Since most of the stationary phases used in this laboratory course are polar in nature, a non-polar solvent would have the lowest eluting power. This means that the compounds to be separated actually can absorb on the column after the solvent was applied.
d. Diethyl ether has a low polarity and therefore also a relatively low boiling point. Under normal circumstances, the solvent elutes of the column very early on (after 1-2 minutes), which means that it does not overlap with the other compounds peaks.
4. a. The equilibrium constant Keq=5 is fairly low in this reaction, which implies that a significant amount of both reactants will remain unreacted, and the yield will be fairly low. In order to improve the yield, one utilizes the Le Châtelier Principle, which states that the equilibrium can be pushed to the product side if one of the reactant is used in excess. In this reaction, the alcohol is used in excess because the carboxylic acids are solids. This way, the alcohol acts as reactant and as a solvent at the same time.
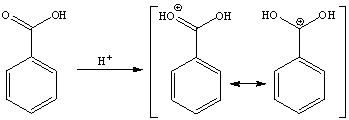
b. The carboxylic acid itself is not a particularly good electrophile. The addition of the sulfuric acid causes the carbonyl group of the carboxylic acid function to be protonated, which makes the carbon atom a better electrophile because the second resonance form (with the positive charge on the carbon atom) because more of a contributor.
c. Despite the addition of the catalyst, the reaction is relatively slow. The reflux increases the temperature in the system and therefore the rate of the reaction. Even then, the reaction takes 60-90 minutes.
d. The addition of water causes a phase separation. While most of the alcohol and the sulfuric acid dissolve in the aqueous layer, the ester and the unreacted carboxylic acid form the organic layer. The addition of the water has to be done after the cooling step to minimize the hydrolysis of the ester.
e. Most of the crude esters still contained some unreacted carboxylic acid and some colored impurities. The distillation step helps to remove these compounds. The vacuum distillation is necessary to minimize the thermal decomposition of the ester during the distillation, which is a significant problem because the esters boil between 200-350 oC.
5. a.

1 2 3 |
The stationary phase is polar (SiO2) and the mobile phase (CH2Cl2:hexane=3:1) is moderately polar. Thus, polar compounds are absorb stronger than non-polar compounds. The locations of the spot on the TLC plate are a result of these polarity differences. The most polar compound moves the least (compound A), the least polar compound (product C) the most. |
b.

1 2 3 |
Since the solvent mixture was not changed, the compounds should show up at the same locations (same Rf-values) as above. However, the spot for compound B disappeared in the product lane because compound B is the limiting reagent in the reaction and the equilibrium constant essentially implies that the reaction consumes the limiting reagent almost completely.
|
6. a. Since the melting point covers the range of the expected melting point, it is very likely that the desired product was isolated. However, the slightly broader melting point (T=3 oC) indicates that the isolated product is either not entirely pure or the skills of the student in terms of melting point determinations are not good (i.e. heating rate too fast). Which impurities are present cannot be determined based on the given information since both mixtures usually show a melting point depression independent from the melting point of the impurity.
b. The saturated sodium bicarbonate solution is usually used to remove acids from the mixture. As a result, carbonic acid is formed which decomposes to form carbon dioxide and water.
![]()
The evolution of carbon dioxide will cause a pressure build-up in the extraction container i.e. centrifuge tube. This is why the procedure asks to vent the container frequently.
c. The o-hydroxybenzaldehyde will have a higher Rf-value because it is less polar than the p-hydroxybenzaldehyde. The ortho derivative exhibits strong intramolecular hydrogen bonding which makes the hydroxyl group accessible to form hydrogen bonding with the polar stationary phase.
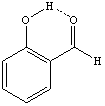
This effect of this intramolecular hydrogen bond is also visible in the IR spectrum and the 1H-NMR spectrum of these compounds.
d. The Diels-Alder reaction carried out in the lab produces three molecules of gas (CO, CO2 and N2). Thus, a larger flask is used to avoid spillage due to foaming, or pressure build-up in the system.
e. Infrared spectroscopy is an absorbance technique, which means that Beer’s law applies here as well. For liquids, a drop of sample between two AgCl plates is enough to form a thin film. Since solids cannot be spread out like this easily, the sample has to be diluted with an optically inert material i.e. KBr. This way, the sample can be thicker without saturating the signal. Ideally, approximately 1-2 % of the sample is suspended in the KBr matrix.
f. The peak at =2350 cm-1 (usually a doublet) is due to carbon dioxide in air. It represents the asymmetric stretching mode for the CO2 molecule. The relative size changes because of the sample concentration and the quality of background correction.
g. When choosing the proper cuvette and solvent, one has to make sure that the cuvette and the solvent do not absorb in the range to be measured. In addition, they cuvette and the solvent have to be compatible with each other. Since polystyrene and polymethacrylate cuvettes start to absorb around 350 cm-1 and also dissolve in many commonly used organic solvents, they are mainly used in the visible range and for aqueous solutions.
h. Extra Credit: The compound contains two bromine atoms. This isotope cluster (1:2:1) is characteristic for that.
7. a. Compound V is relatively non-polar because it dissolves well in heptane, which is a non-polar solvent and poorly in water, which is a polar solvent.
b. The best solvent for recrystallization here is isopropanol because the solubility curve for compound V is pretty steep and the medium polar impurity should dissolve fairly well in this solvent as well.
c. In order to dissolve 4.0 g of compound V, the student will need 40 mL of heptane (=4.0 g/(10.0 g/100 mL)) at T=100 oC. At 0 oC, 1.6 g (=40 mL* 4.0 g/100 mL) remain in solution, and 2.4 g (=4.0 g – 1.6 g) will precipitate.
d. If the melting point of the compound is below the boiling point of the solvent, the compound is heated above its melting point. In many cases, this causes the compound to form an oil rather than a crystalline solid when the mixture is allowed to cool down.
8. Spectrum 1: Compound F
Characteristic peaks at cm-1: 2872-2982 (CH, sp3), 1720 (C=O, ester), 1610 (C=C, aromatic), 1465, 1367 (CH2, CH3, bend), 1280, 1107 (COC, ester)
Spectrum 2: Compound K
Characteristic peaks at cm-1: 3341 (OH, alcohol), 3020 (CH, sp2), 2874-2931 (CH, sp3), 1671 (C=C, alkene), 1456 (CH2, CH3, bend), 1006 (C-OH), 970 (oop., trans alkene)
Spectrum 3: Compound H
Characteristic peaks at cm-1: 2872-2976 (CH, sp3), 1762&1809 (C=O, anhydride), 1369, 1467 (CH2, CH3, bend), 1077, 1166 (COC)
Spectrum 4: Compound I
Characteristic peaks at cm-1: 2875-2952 (CH, sp3), 1718 (C=O, ketone), 1360, 1457 (CH3, bend)
Spectrum 5: Compound C
Characteristic peaks at cm-1: 3001, 3081 (CH, sp2), 2847-2981 (CH, sp3), 1643 (C=C, alkene), 1440 (CH2, bend), 913, 994 (oop, mono-subst. alkene)
Spectrum 6: Compound A
Characteristic peaks at cm-1: 2912-2999 (CH, sp3), 1396, 1556 (NO2), 1367, 1439 (CH2, CH3, bend)
Spectrum 7: Compound L
Characteristic peaks at cm-1: 3311 (CH, sp, alkyne), 2873, 2961 (CH, sp3), 2119 (C=C), 1387, 1454 (CH2, CH3, bend), 631 (CH, bend, alkyne)
Spectrum 8: Compound E
Characteristic peaks at cm-1: 3278, 3366 (NH2, prim. amine), 2864-2924 (CH, sp3), 1606 (NH2, scissoring), 1382, 1450 (CH2, CH3, bend)
9. a. Degree of unsaturation: D.U.= (2*C+2-H+N)/2 = (2*10+2-13+1)/2 = 5
b. The most important peaks are at (in cm-1): 3344 (NH), 2917 (CH, sp3), 1720 (C=O), 1538, 1616 (C=C, aromatic), 1156, 1251 (COC)
c. The 13C-NMR spectrum shows seven peaks, which means that the target molecule has to be fairly symmetric because the molecular formula shows ten carbon atoms.
d. The 1H-NMR spectrum shows four signals, all of them being singlets. The signals at 6.73 ppm (2H) and 6.82 ppm (1H) are due to two different type hydrogen on the benzene ring which do not have any direct neighbors. Overall, there is a trisubstitution on the ring. The signals at 2.86 ppm (3H) and 2.29 ppm (6H) are due to one or two isolated methyl groups attached to a benzene ring or a carbonyl group, or to an electronegative atom like nitrogen.
e. The 13C-NMR spectrum exhibits seven signals. The signals at 139, 151 and 156 ppm are due to quaternary carbon atoms. Later one is due to the carbonyl function in the molecule. The signals at 119 and 127 ppm are due to CH functions from the benzene ring. The signals at 21 and 28 ppm are a result of two methyl groups.
f. Based on the discussion above, compound W is 3,5-dimethylphenyl methyl carbamate (or “XMC”).

Note: The methyl group in 4-position and the aldehyde function are switched the adjacent hydrogen atoms would be shifted to ~8 ppm due to the proximity of the oxygen.