Condensed answer key for Final Chem 30 CL (Fall 2001)
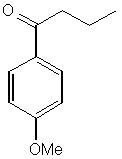
1.

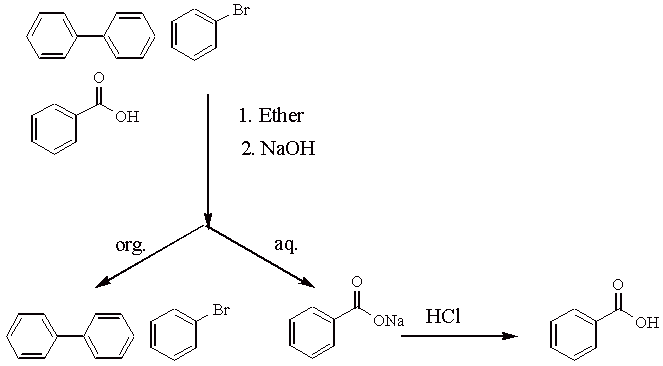
2.
a. A higher temperature makes it more likely to obtain polynitration products that are explosive.
b. It serves as a solvent for the methyl benzoate. The carbonyl group gets protonated!
3.
a. No, water has acidic protons
b. No, isopropanol has acidic protons
c. No, nucleophilic substitution
d. No, reaction of carbonyl group
e. No, doesn't react, but also doesn't solvate the Grignard
f. Yes, ether that can solvate the Grignard
4. Cinnamon aldehyde possesses trans-conformation in regard to the double bond. This stereochemistry remains intact because there is no radical chemistry involved in this reaction. The only isomers that can be obtained from the reaction are trans, trans and trans, cis.
5. The amide shows a partial C-N-double character. At low temperature, the methylene groups are not equivalent because they see magnetically different environments (like in an alkene). At higher temperatures, the rotational barrier is overcome and the NMR instrument sees the average value because the amine group freely rotates and the methylene protons become equivalent.
6.
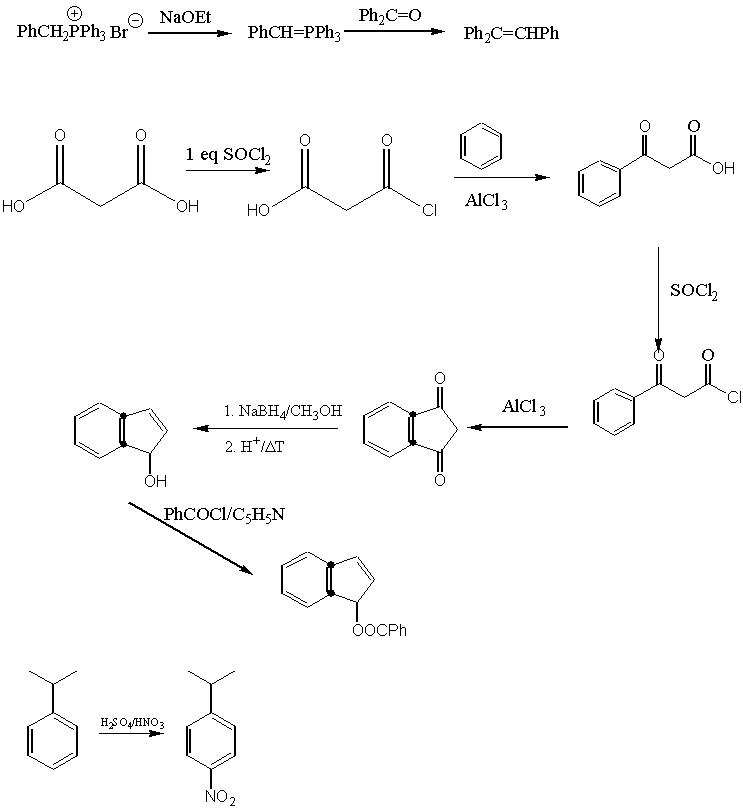
7.
a. AlCl3 forms an adduct with the formed ketone, which ties up one equivalent of the 'catalyst'.
b. Friedel-Crafts-Alkylation: Product is more reactive than starting material
Friedel-Crafts-Acylation: Product is less reactive than starting material and acylium ion is a weak electrophile (resonance)
Nitration: Product is less reactive than starting material and nitrinium ion is a strong electrophile
c. One equivalent is necessary to form the amide, the other one to tie up the formed HCl (as ammonium salt)
d. The acid chloride is more reactive than the acid itself. Therefore, the reaction can be carried out at room temperature.
8.
The starting material contained significant amounts of water. Water is formed in the equilibrium. The presence of water shifts the equilibrium to the left side, which results in lower yields of the ester (Le Chatelier Principle).
9.
- The reaction is entropy driven (heat promotes teh reaction)
- The second step is intramolecular
- The high degree of conjugation means a high thermodynamical stability
10.
a. Rf value increases because the compound dissolves better in the mobile phase
b. Rf value increases because the compound interacts less with stationary phase
c. Rf value increases because not all of the material is absorbed and is 'washed away' in the beginning (tailing)
11.