FINAL EXAMINATION
Organic Chemistry 30CL
Instructor: Dr. Bacher
December 7, 2001
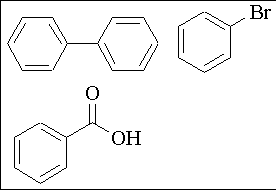
1. (15 points) Outline a separation scheme for the isolation of benzoic acid from ether, biphenyl and bromobenzene (use any needed solvent and reagents).

2. (8 points) In your nitration experiment
a. Why do you have to cool the reaction mixture with an ice bath? Show appropriate diagrams and chemical equations.
b. Why do you have to add the sulfuric acid to the methyl benzoate first?
3. (18 points) Explain briefly if the following solvents would be or would not be a good choice as solvent for a Grignard reaction.
a. water
b. isopropanol
c. chloroform
d. 2-butanone
e. petroleum ether
f. di(n-propyl)ether
4. (6 points) Explain briefly why the cis,cis-isomer is not formed in the Wittig reaction in the lab?
5. (12 points) N,N-diethyl-m-toluamide shows two triplets and two quartets for the methylene and the methyl group in the H-NMR spectrum at 9 oC. At 35 oC, the methyl signals coales into one broad signal. At 44 oC, the same happens to the signals for the methylene groups. At 85 oC, a fully resolved triplet and a quartet can be observed. Explain this observation.
6. (27 points) Predict the major products for the following reactions (including the correct stereochemistry!).
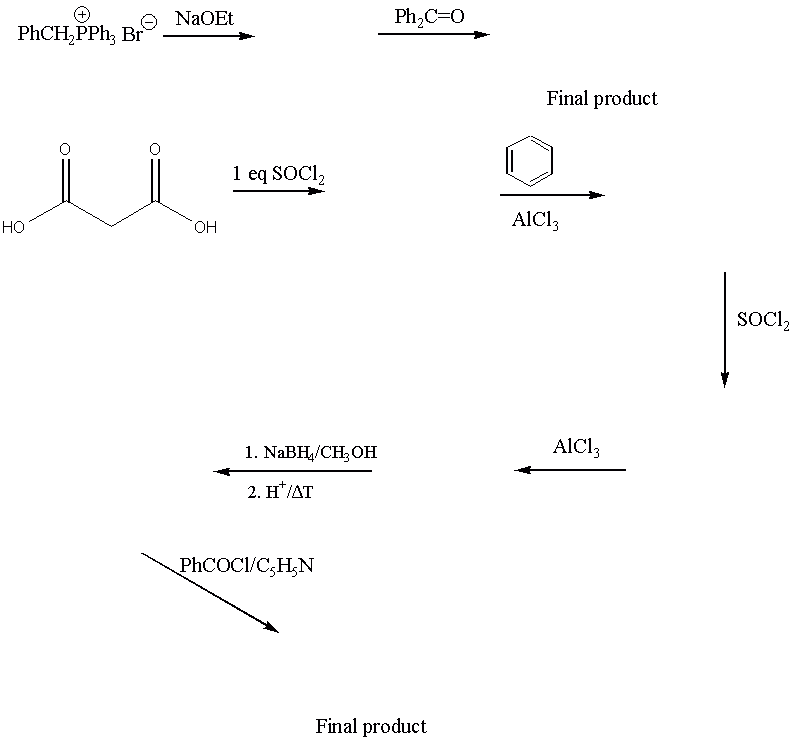
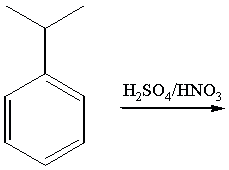
7. (20 points) Answer the following questions briefly, showing pertinent equations and diagrams.
a. In the FC-acylation, why do you have to add more than one equivalent of aluminum trichloride although it is said to be the catalyst?
b. While a FC-alkylation and nitration leads to multiple substitutions, while the FC acylation does only afford mono-substitution.
c. Why do you need two equivalents of diethylamine in the formation of the amide?
d. Why did you convert the toluic acid into the acid chloride?
8. (10 points) A student obtains a 10% yield in the esterfication reaction of benzoic acid. How can one rationalize this poor yield?
9. (12 points) Which factors drive aldol reaction forward (towards the formation of the tetraphenyl-cyclopentadienone)?
10. (12 points) Explain briefly how is the Rf-value of a polar compound is influenced if
a. a more polar solvent is used to develop the chromatogram
b. the surface of the TLC plate is treated with non-polar silane
c. the student spots too much material on the TLC plate
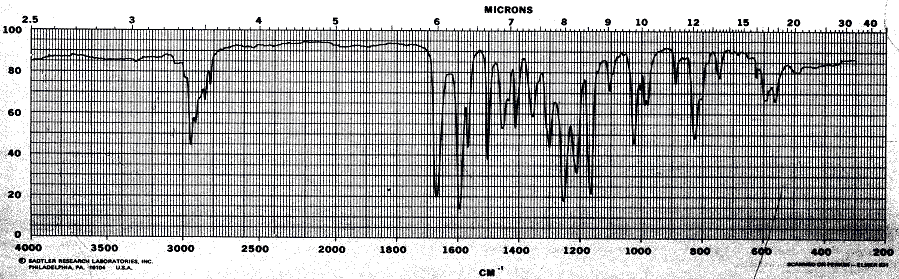
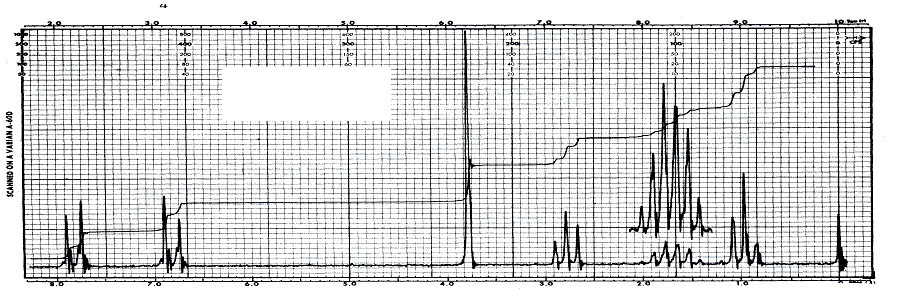
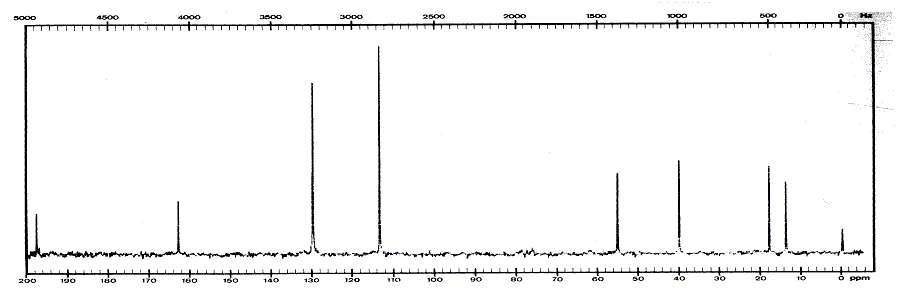
11. (20 points) Compound X has a molecular weight of 178.23 g/mol. It contains only carbon, hydrogen and oxygen and 27 atoms total. Given are the following spectra. Suggest a structure for compound X.