Effect of R on the cis-trans ratio in the epoxidation of cis-cinnamates

| R-group | cis/trans |
| OCH3 | 11.7 |
| CH3 | 7 |
| H | 5.7 |
| CF3 | 0.8 |
| NO2 | 0.27 |
Conclusion:
Electron-withdrawing substituents increase the formation of trans epoxides, because they can stabilize the radical intermediate.
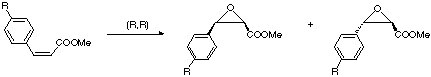
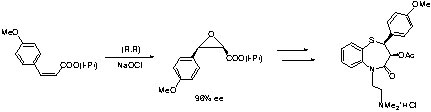
Example: Synthesis of diltiazem (anti-hypertensive agent)
Reactivity of conjugated polyenes
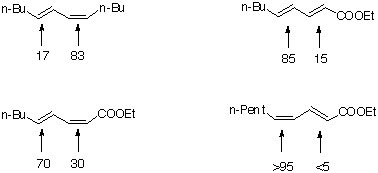
Conclusion:
High regioselectivity due to
1. cis-double bonds are more reactive than trans- double bonds
2. electron-withdrawing groups slow down the epoxidation at the appropriate double bond.