
Week 6 Condensed Answer Key
1. Catalyst. Without sulfuric acid the reaction rate would be too slow.
2. Organic components present: acetic acid, isopentyl acetate and isopentyl alcohol
3. 5% sodium bicarbonate is used in the work-up. This wash neutralizes the reaction (removes the H2SO4), efficiently removes the excess acetic acid and also extracts out any remaining isopentyl alcohol .
4.

5.
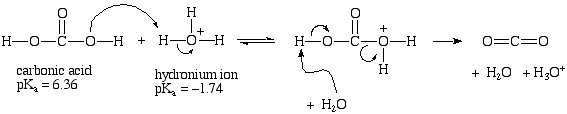
**QUESTIONS FROM LANDGREBE**
7. Using equation 10 from the text (page 131 of Landgrebe):
 |
Clarification: V2 is the volume of the extracting solvent used for each extraction. Thus, if 5 extractions were carried out using 1 mL of solvent 2 each time, then V2 = 1 mL. |
thus,
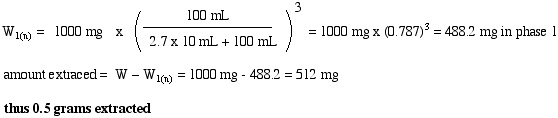
8.
0.10W = W (v1 / ((K • v2) +v1))n
Since v1 = v2 and K = 0.5; assign v1 = 1 unit volume
0.10W = W (1 / ((0.5 (1) +1))n = W (0.666)n
thus,
0.10W = W (0.666)n
0.10 = (0.666)n
n = 5.6
6 extractions is needed
11. Add NaCl (salting out). Salting out increases the partition coefficient of many organic compounds between the organic and aqueous layer. Water molecules better solvate the sodium chloride ions over the organic molecules (why? like-dissolves-like rule).