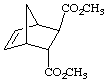
Week 4 Problem set - CONDENSED KEY
1. a) the time required for the compound to pass through the column and reach the detector
b) increase
c) decrease
d) decrease
2. Cyclopentanol is more polar than cyclopentanone. Thus the cyclopentanol would spend more time in the stationary phase.
3. Yes. The retention time for cyclopentanol would decrease and for cyclopentanone it would increase.
4. Polarity is not an issue here since diethyl ether has a significantly lower boiling point than borneol.
5. The compounds need time to interact with the stationary phase for separation to occur. If they pass through the column too fast (because of too high of a flow rate) or if the column temp is too high (so that the compounds always stay in the gas phase) then the compounds will never interact with the stationary phase.
6. From course reader: a volatile, flammable fraction of petroleum ether, obtained by distillation from crude oil. Petroleum ether consist of C5H12 and C6H14 hydrocarbon isomers (e.g., pentane, 2-methylbutane, 2,2-dimethylpropane, hexane, 2-methylpentane, etc.). There are two types. There is low boiling point petroleum ether (bp 30-60°C) and high boiling point petroleum ether (bp 60-90°C). High boiling point petroleum ether and ligroin are synonymous. Thus, the fraction collected between 60-90 °C is ligroin. Ligroin consists mosly of C6H14 hydrocarbon isomers.
FYI: Do not be fooled by the "ether" term in "Petroleum ether". Petroleum ether is NOT an ether. There are no oxygen containing compounds present.
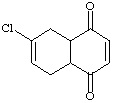
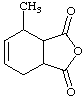
7.
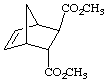 |
 |
 |
8. The s-cis conformation of the diene is required for a Diels-Alder reaction. In reaction 2, the s-cis conformation is highly unfavored due to the steric interaction of the tert-butyl groups. In reaction 1, the diene is essentially locked into the s-cis conformation.

9.
| a.
|
b. 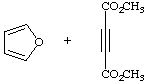 |