

endo approach
(Pro-R face)
exo approach
(Pro-S face)
Week 3 Problem set - CONDENSED KEY
1.
The LUMO for camphor:
 |
 |
| Camphor, LUMO endo approach (Pro-R face) |
Camphor, LUMO exo approach (Pro-S face) |
The exo approach is more sterically encumbered.
The LUMO for 2-norboranone.
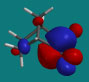 |
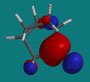 |
norboranone3.jpg) |
| Camphor, LUMO lateral view |
Camphor, LUMO endo approach |
Camphor, LUMO exo approach |
The endo approach is more sterically encumbered.
| 2. a) | NaBH4 |
| b) | endo isomer since exo hydride approach is least sterically hindered |
| c) | antithesis of previous explanation |
| d) | exo is formed (if the hydrogen is endo then the OH has to be exo) |
3. see reader and lecture notes
4. yes; contains equal quantities of R and S forms.5. 200 mg (5.350 mmoles) of NaBH4 and 200 mg (1.305 mmoles) of camphor each mole of NaBH4 has 4 equivalents of hydride thus only (1.305/4) mmoles of NaBH4 is needed. Thus 5.350 - (1.305/4) = 5.02 mmoles of NaBH4 in excess