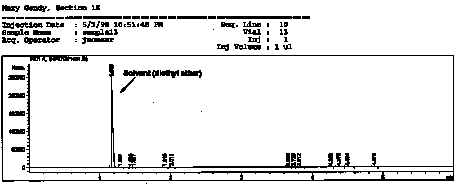
| This is a chromatogram obtained from a student's sample for the reduction of camphor experiment. Note that the largest peak in the is the solvent peak and that the other peaks, relative to the solvent peak, are very very small.
NOTE: this is the same chromatogram figures given in my reader. See GC appendix in the back of my reader for better clarity of figures. |
 |
| This is an expansion of the above chromatogram, excluding the solvent peak. Now the other peaks are visible. | 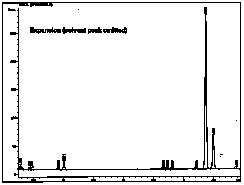 |
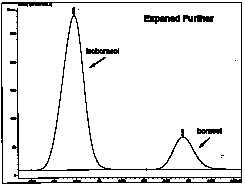
| The chromatogram is further expanded only showing the isoborneol and borneol peaks. The retention times are 4.375 and 4.494 mins, respectively. [Note: The retention times for isoborneol and borneol in your chromatogram will most likely not be the same values as in this example. Retention times are very sensitive to the conditions (i.e., flow rate of carrier gas, column temperature, sample size, etc.) used at the time the gc was performed. Definition: Retention time is the time required for a compound to come off the column (that is, the time it takes to travel from the injection chamber to the detector). |
 |
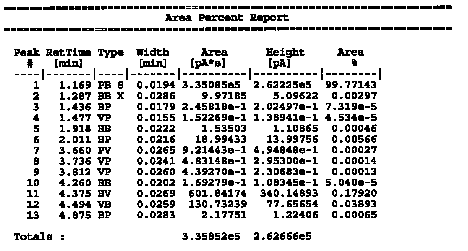
| The data table represents the peaks from the above chromatogram in tabular form. The retention time is given for all the peaks. In addition, the Area is also given. Note that peak #1 is the solvent peak and has the great area. Peaks #11 and #12 are the isoborneol and borneol peaks, respectively. All other peaks present are trace and not of interest.
Calculation of the % isoborneol and % borneol % isoborneol = [602 / (602 + 131)] x 100 = 82.1 % % borneol = [131 / (602 + 131)] x 100 = 17.9% |
 |