Note: Commentaries are in color and placed in brackets.
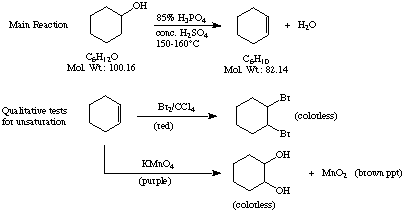
Experiment 15: April 3, 1998 Afshin Farahi [date and signature] Purpose: Cyclohexene will be prepared from cyclohexanol. The product will be isolated by simple distillation. In addition, as a qualitative test for unsaturation, the reactivity of cyclohexene with KMNO4 and Br2 will observed. Reaction Schemes:

Physical Properties: [obtain physical properties from Aldrich Catalog in the reference section of the library]
Substance |
mw |
amount |
mmol |
mp(°C) |
bp(°C) |
density g/mL |
Solubility water/organics |
| Cyclohexanol | 100.2 | 1.3 mL | 12 | 20-22 | 160-161 | 0.948 | no/yes |
| Cyclohexene | 82.2 |
|
|
|
83 |
|
no/yes |
| 85% H3PO4 |
|
0.4 mL |
|
|
|
|
yes/no |
| Conc. H2SO4 |
|
6 drops |
|
|
|
|
yes/yes |
[note: both H3PO4 and H2SO4 are catalysis and thus mmol used, mw, mp, and bp are not significant]
Safety Considerations: Both H3PO4 and H2SO4 are corrosive and should avoid contact with skin. Bromine is highly corrosive and CCl4 is a suspect carcinogen; use only in fume hood and avoid contact. 5% aqueous sodium thiosulfate should be used for treatment of bromine burns. [obtained from text and lecture; see special instructions section for exp.] Waste Disposal Considerations: Organic residues are to be discarded in the designated nonhalogenated waste bottle. However, solutions remaining after the bromine test should be placed in the halogenated waste bottle. The KMNO4 waste should be discarded in a bottle labeled heavy metal waste. [obtained from text; see waste disposal section of exp.]
|
Procedure Outline: Apparatus Assembly
Dehydration
Isolation and Drying of Product
Distillation
IR Spectrum
Unsaturation Test
|
Observations and variations:
cyclohexanol solidified in dispensing bottle; had to warm bottle in hot water bath
Increased hotplate temp. to 180°C Distillation took 40 mins.
Used 1.5 mL of aq. NaCl soln.
Organic layer was cloudy
Dried 5 mins
Trace amount of solid transferred over
increased temp. to 180°C
tared vial = 20.982 g sample + vial = 21.340 g
obtained IR of sample on silver chloride plates
[place your observations for unsaturation tests here] |
Results: [show all calculations] Cyclohexanol is limiting reagent thus,
Cyclohexene isolated
mass cyclohexene = 21.340g-20.982g = 0.358 g thus,
Experimental Yield
IR Data Table (neat): [in this example the IR spectrum is not included; however, you need to include an IR spectrum of your compound]
| Wavenumber (cm-1) | Assignment | Comments |
3024 2934 1662 718 |
=CH unsaturated stretching CH saturated stretching CH2 bending =CH out-of-plane bending |
weak intensity |