MIDTERM I, 14C
MOUSER, July 8
1.
a. 2-methylpropene or isobutylene
b. (E)-2-pentene or trans-2-pentene
c. 3-pentene-2-ol or (S)-3-hydroxy-2-pentene
d. 2,4,6-tribromophenol or 1,3,5-tribromo-2-hydroxyphenol(R)-2-cyclohexen-1-ol
e. anisole or methyl phenyl ether or methoxybenzene
f. o-nitrobenzoic acid or 2-nitrobenzoic acid
g. m-allylstyrene or 1-allyl-3-vinylbenzene
2.
a. 5
b. 3
c. 2
d. s bond of the C=C is overlap of sp2-sp2 and for the C–C is overlap of sp2-sp2
e. C–C bond
3.
a. all atoms are 0 with exception to N which = +1
b. C of C=O is sp2; C of methyl is sp3; N is sp3 and O is sp2
4.
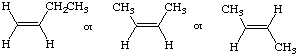
a. 
middle structure is highest; sterics (torsoinal strain)
b. 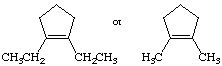
fisrt stucture is highest; sterics (torsoinal strain)
c. 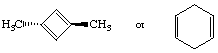
first structure is highest; antiaromatic
d. 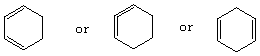
middle strucutre is highest; ring strain (sp carbon would prefer to be linear)
e. ![]()
Middle strucuture is highest; ring strain and least subst. (this one has 2 sp2 carbons going to sp3 and in addition it is disubstituted)
5.
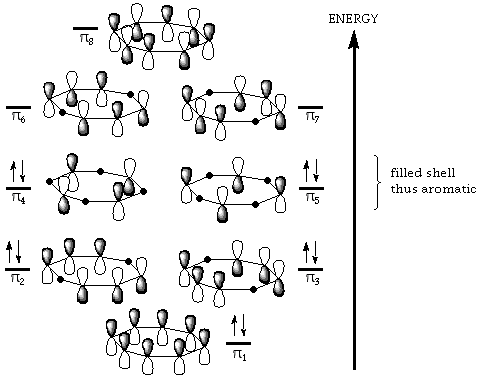
¹-Molecular Orbitals
6.
| not aromatic | antiaromatic | aromatic |
| aromatic | antiaromatic | aromatic |
| aromatic | aromatic | aromatic |
7. Structure I has a large dipole since resonance structure IA is aromatic! (and thus a strong contributor). Note that structure IIB is antiaromatic (if we assume it is planar).
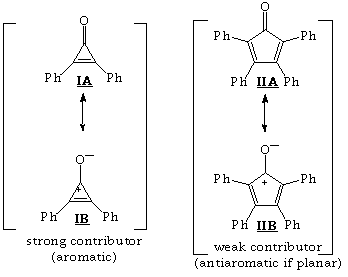
8. From pp. 746-747 of text.

9. Benzene’s is aromatic and thus the pi electrons a delocalized. Since they are delocalized they are equally shared between all the C atoms. Cyclooctatetraene is nonaromatic and thus the pi bonds are localized.
10. See lecture note for MO diagram for 1,3-butadiene.
11.
a. yes
b. 3 x C2, 1 x C3
c. 3 x s
d. S2 or S6 (full credit if you identified one or more)
e. yes
12.
a. yes
b. 1 x C2
c. 1 x s
d. S2 (full credit if you identified one or more)
e. yes