
1.
| a. 3-methylbutanal |
| b. 1-(4-ethoxyphenyl)-1-propanone
1-ethoxy-4-(1-propanoyl)benzene or (4-ethoxyphenyl) ethyl ketone |
| c. 3-methyl-2-cyclohexenecarbaldehyde |
| d. (Z)-2-methyl-2-butenal
if cis -2 pts |
| e. 1-ethyl-3-cyclopentenecarboxylic acid(R)-2-cyclohexen-1-ol |
| f. HO—CH2CH2CH2CO2H |
| g. CH3C(O)CH2CH2CH2CO2H |
2. 80°C, 98°C, 36°C
3. 8, 2, 5
4. 3, 3
3, 7
5. for deuterium I = 1; Multiplicity = 2nI+1 = 2 x 2 x 1 + 1 = 5 peaks, quintet
6.

thus, the conjugate base of acetone is more stable than the conjugate base of ethane.
7.

a stronger acid and a stronger base on the reactant's side of the equation favors the forward direction (or reaction to the right).
8. B is considerably more acidic; the conjugate base of B is more stable. The cyclopentatrienyl anion is not aromatic. The cyclopentadienyl anion is aromatic. (Show structures for full credit).
9.
![]()
10.

Dilute sample: A sharp O—H signal is expected under dilute conditions. (Hydrogen bonding with another alcohol is unlikely under dilute conditions either because of spatial separation (gas phase) or solvent separation.)
Neat sample: A broad O—H signal is expected resulting from hydrogen bonding between molecules. However, due to the nearby bulky tert-butyl groups, hydrogen bonding interactions between molecules is sterically encumbered.
11. water, 16; amine 38; ketone 19-20; alcohol 16-19; ketone 19-20; alkyne 25
12. C5H9BrO; Exact Mass: 163.9837
13. a. 134 peak
b. 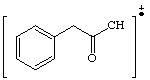
c. 91 peak
d. 
14. B, E, D, G
15.
![]()
IR Spectrum:
A, =C—H stretch
B, C—H stretch
C, aromatic overtones
D, C=C alkene stretch
E, C=C aromatic stretch
F, C—O stretch
Proton NMR:
A & B, Aromatic
C, O—CH2—CH=CH2
D, O— CH2—CH=CH2
E, O— CH2—CH=CH2
Carbon NMR:
A, ipso aryl carbon
B, O—CH2—CH=CH2
C, O—CH2—CH=CH2
D, O—CH2—CH=CH2
16. ![]()
17.
