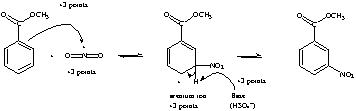
1. CO, CO2, N2
2. To synthesize and study the structure of sterically congested polycylic aromatic compounds.
or
To study the peri interactions and the resulting distortions in the acene cores.
[if only synthesis is mentioned then +3 points and only if structures are given.]
3. TRUE
4. 63° (±5°) distortion
NO PARTIAL CREDIT
5. All the phenyls freely rotate with the exception the two phenyls at the 9,10 position (on a NMR time scale). Should show their math. 4, 4, 6 for the phenyls and 4 for the acene core.
[IF all the phenyls freely rotate then 16 signals would be expected]
[IF all the phenyls DO NOT freely rotate then 22 signals would be expected]
6. HCl is a product. Thus, if only one mole of diethylamine is then the reaction will not go to completion (at best only 50%).
HCl + NH(CH2CH3)2 ---------> +NH2(CH2CH3)2
7. SOCl2 + H2O ------> SO2 + HCl
Ph-C(O)Cl + H2O ------> meta-toluic acid
8. PhMgBr + CH3CH2OH ------> Ph-H + + CH3CH2OMgBr
9. Pentane is not polar enough to dissolve the Phenyl Grignard and thus is a poor solvent. In pentane the Phenyl Grignard will remain at the surface of the magnesium metal disallowing further reaction (since the metal surface is no longer exposed).
10. absolute ethanol was the solvent for the reaction; 95% ethanol was uses to rinse impurities clinging to the product surface
11. Since water is a product of the aldol reaction, minimization of the presence of water will help drive the reaction to the right (Le Châtelier's principle).
12. a. Concentrated H2SO4. (-2 for not mentioning concentrated).
b. strong acid so carbonyl is protonated (like-dissolves-like rule).
13.
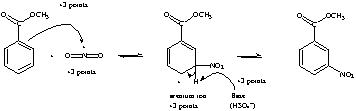
14. a. (3 points) Peak at 83
b. (3 points) Peak at 98
c. (3 points) C7H14
d. 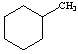
e.
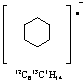
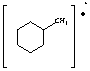
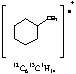
14. Ring strain and localized double bond (isolated double bond). Need to supply structure for full credit.
15. acylates at the 4 position; no reaction; alpha
16. 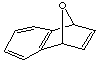 ; hexaphenylbenzene
; hexaphenylbenzene