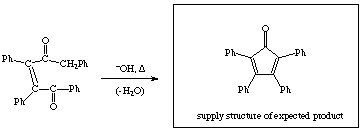
1.Water is a product of the reaction so any added water will shift the equilibrium towards the reactants (show reaction equation for full credit.)
2.
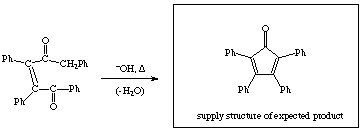
3.
4. Water is a product of the reaction so any added water will shift the equilibrium towards the reactants. [+6 points for explanation]
![]() [+4 points for rxn equation]
[+4 points for rxn equation]
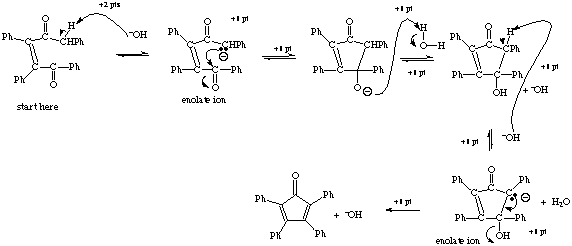
5. ![]() [+5 points for explanation]
[+5 points for explanation]
at 95°C the Total pressure = 634 + 118 = 752 mm Hg; at 96°C the Total pressure = 658 + 122 = 780 mm Hg; thus, the bromobenzene steam distills at ~ 95°C (or 95-96°C). [+5 points for correct answer]
6. No. Acetic acid is miscible with water in order for ![]() to hold true.
to hold true.
7. a.
![]()
b. Though both X and Y are trisubstituted alkenes, X is favored. The ring expansion is favored due to less angle strain in the five-membered ring.
8.
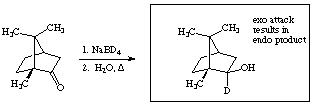
9. a. LOW. The compounds need time to interact with the stationary phase in order to separate. If the temperature is too high then the compound spend most of their time in the gas phase thus minimizing their interaction with the stationary phase
b. LOW. Passing the components through the column too fast will minimize their interaction with the stationary phase.
c. The stationary phase is the liquid phase. Separation of gaseous components occurs through interaction with the liquid phase.
10.Spot #1 Rf = 0.95; Spot #2 Rf = 0.48
11. a. water; benzoic acid has a high solubility in water at high temperature and a low solubility at low temperature (steep solubility curve).
b. Dissolve 136 mg of benzoic acid in 2 mL of boiling water. Once all of the benzoic acid has dissolved then slowly cool the solution to room temperature. Place on ice if necessary.
12.
|
|
|
|
|
|
13. a.(4 points) 4-hydoxybutyric acid (or gamma-hydoxybutyric acid = GHB)
b. The above reaction is intramolecular. Thus the alcohol moiety is in high concentration since it is tethered to the carboxylic acid group (i.e., infinite concentration). The experiment reaction carried out in lab was an example of an intermolecular process.
14.
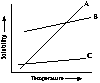
The product has a high solubility in diethyl acetate (see line B in graph) and a low solubility in ligroin (line C in graph). However, when the mixed solvent system results in a steep solubility (see line A) which is optimal for crystallization.
If only ligroin had been used the product would have crashed out of solution upon formation. If diethyl acetate had been used the product would on never recrystallized.
15. 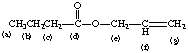
a. starting from left side: =C–H stretch, C—H stretch, C=O stretch, C=C stretch, C–O stretch
b. starting from left side: d, {f,g}, e, {c,b,a}