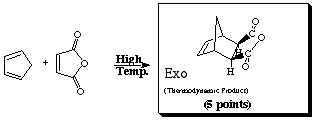
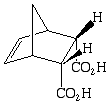
sterically less congested than the endo
6.
1.
1) Dissolve in solvent (diethyl ether or methylene chloride) +2
2) Extract organic layer with 5% NaOH +5 (+2 if NaHCO3)
3) Isolate aqueous layer and neutralize with acid +2
4) Extract aqueous layer with diethyl ether +2
5) Dry organic layer over anhydrous Na2SO4 +2
6) Evaporate off diethyl ether +2
if suction filtration is mentioned, -5 points
2. (760-560)/560 = 200/560 = 20/56 = 5/14; thus 5 moles Eugenol/14 mole water; or 0.357 mole of Eugenol/mole of water
3. DELETED from exam
4. 3 signals, 6 signals
7 signals, 8 signals
5.

sterically less congested than the endo
6.
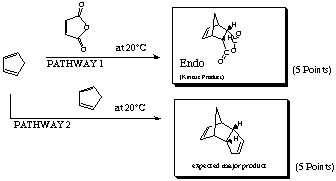
PATHWAY 1 is favored; the HOMO of the cyclopentadiene is closest in energy to the LUMO of the maleic anhydride.
6.
both the endo and exo are possible. However, the endo is favored due to secondary orbital overlap which results in a more stable transition state.
need to show LUMO and HOMO and secondary orbital overlap for full credit.
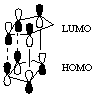
(dotted line is secondary orbital overlap and solid line indicated bond formation)
7. = 1.4534 - (3x0.00045) = 1.4520(5)
8. a) neutralize reaction mixture (kill the catalysis)
b) remove excess acetic acid CH3CO2H + NaHCO3 --> CH3CO2– + CO2 (g) + H2O
c) remove isopentyl alcohol -3 point is they show ROH going to RO-
9. (10 points) Below, supply a sketch of a Hirsch funnel.
10.
11. B, E, D, G
12.
a) the time required for the compound to pass through the column and reach the detector
b) decrease
c) increase
d) increase (or approx double)
13. In order for separation to occur, the compounds need to interact with the stationary phase. If they pass through the column too fast (because of too high of a flow rate) or if the column temp is too high (so that the compounds always stay in the gas phase) then this minimizes the interaction of the compounds with the stationary phase. This results in poor or no separation of the compounds present
14. Yes, the compounds need to associate (interact) with the stationary phase for separation to occur.
NO, if the compounds do not interact with the plate then the length doesn't matter
15.
a. 6
b. alkene, carbonyl
c. credit given for any answer with justification
d. Stretches: H-C=, H-C, O=C, C=C, C-O; bends: H-C
e. in order of decreasing ppm: C,CH,CH2,CH, CH
Compound Z