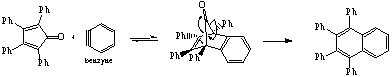
1. Water is a product of the reaction so any added water will shift the equilibrium towards the reactants (show reaction equation)
2.
+ 4 pts for intermediate structure
+2 pts for each arrow
3. a.
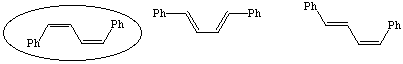
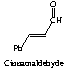
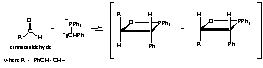
Cinnamaldehyde is trans. The R group's double bond remains intact throughout the reaction and thus the does not isomerize. Thus one trans bond is expected in the final product. (cis,cis)1,4-diphenyl-1,3-butadiene would not be an expected product.
b. the (trans.trans)-isomer has more exposed surface area to interact with the stationary phase.
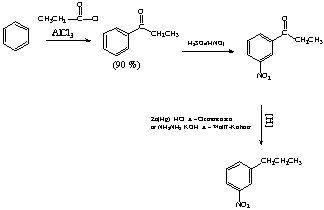
4. (15 Points) Propose a reasonable synthesis for 1-nitro-3-propylbenzene starting from benzene and any needed reagents.
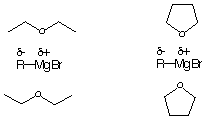
5. (10 points) The phenyl Grignard produced in the Grignard experiment would be more soluble in which solvent (circle): diethyl ether or THF (tetrahydrofuran)? Explain (give two reasons).
a) polarity - THF more polar.
b) less crowding (sterics) - the oxygen is more expose and thus the can better stabilize the Phenyl Grignard
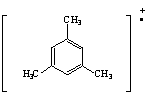
6.
a. Linear
b. Resonance structure I is favored due to the octet rule. Structure I's acylium carbon is sp hybridized which is linear (180° bond angle).
c. A typical C=O is 1.22 angstroms and CO triple bond is 1.12 angstroms. PC_Spartan predicted a CO bond distance of 1.15 angstroms (your value may of differed slightly) for the acylium ion which is closest to the CO triple bond value.
7.
8. Both acetyl chloride and aluminum trichloride react violently with water.
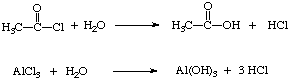
9.
a) These were the targets.
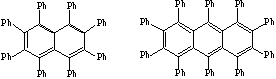
b) Purpose was to examine structure of the acene ring. In both structures the acene rings were distorted. The decaphenylanthracene showed the greatest distortion. The peri phenyls groups were most likely responsible for the twisted acene ring.
c) This twisting may significantly influence the aromaticity of these compounds since a planar ring system, which contains the pi-bonds, is necessary for good overlap.