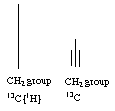Organic Chemistry 130AL
Instructor: Mouser
August 3, 1999
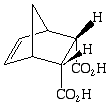
1. (15 points) Caryophyllene is a common contaminant present in Eugenol (when isolate from cloves using steam distillation). Outline a separation scheme for the isolation of pure dry Eugenol (use any needed solvent and reagents). Note: GC is not a practical means to purify a bulk sample.
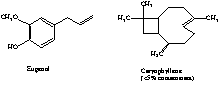
1) Dissolve in solvent (diethyl ether or methylene chloride) +2
2) Extract organic layer with 5% NaOH +5 (+2 if NaHCO3)
3) Isolate aqueous layer and neutralize with acid +2
4) Extract aqueous layer with diethyl ether +2
5) Dry organic layer over anhydrous Na2SO4 +2
6) Evaporate off diethyl ether +2
if suction filtration is mentioned, -5 points
2. (10 points) If Eugenol and water codistill at 91.6 °C at atmospheric pressure (760 mm Hg). Given that the vapor pressure of water at 91.6 °C is 560 mm Hg, calculate how many moles of Eugenol codistills with every mole of water. Show all calculations.
(760-560)/560 = 200/560 = 20/56 = 5/14; thus 5 moles Eugenol/14 mole water; or 0.357 mole of Eugenol/mole of water
3. (15 points) In the esterification reaction, an aqueous sodium bicarbonate extraction was carried out. What role(s) did the aqueous sodium bicarbonate extraction serve in the isolation of isopentyl acetate (show any appropriate reaction equations or diagrams)?
a) neutralize reaction mixture (kill the catalysis)
b) remove excess acetic acid CH3CO2H + NaHCO3 --> CH3CO2— + CO2 (g) + H2O
c) remove isopentyl alcohol -3 point is they show ROH going to RO-
4. a. (6 points) If a 1.0 mL aqueous solution contained 100 mg of Compound X and was extracted with 4.0 mL of CH2Cl2, how much X would be in the organic layer?
Given: Solubility of X in water at room temperature = 100 mg/mL
Solubility of X in CH2Cl2 at room temperature = 100 mg/mL
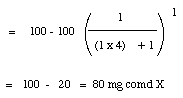
?b.?(9 points) Would if instead, the solution was extracted with four 1.00 mL portions of CH2Cl2, how much of X would be in the combined organic layers organic layers?
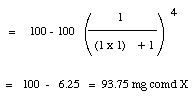
5. (10 points) The melting point of an unknown compound was determined to be between 163-165 °C. The unknown was thought to be one of three known compounds which have very similar melting points. Without the aid of spectroscopic techniques (gc and TLC are also not allowed), explain how the correct compound could be simply identified. Assume all of the compounds are available).
MIXED melting Point; thus mix the unknown with a known; if the mp does not change then they are the same compounds.
6. (16 points) How many 13C{1H} NMR signals would you expect to see for the following compounds (circle your answers)?