
130BL FINAL EXAM
Instructor: Dr. John K.M. Mouser
June 7th, 1999, Winter Quarter
1.
(1.0 g) / (210 g/mole) = 4.76x10-3 moles
4.76x10-3 moles x 22.4 liters/mole = 0.107 L
Thus, 0.11 liters or 110 mL.
2.

3. One set of electrons is orthogonal to the aryl ring and cannot participate in the pi system. Thus the aryl ring consist of 6 pi electrons which is a Hückel number.
4. SO2 and 2 HCl
5. HCl and acetic acid
6. Exact Mass = 181.0375
7.
a. Mol. Wt.: 177.08 (not Exact Mas =176.0401
b. C7H13Br
c. 176 m/z peak
d.
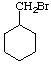
e.
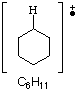 Peak at 83 |
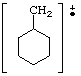 Peak at 97 |
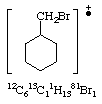 Peak at 179 |