Instructor: Dr. John K.M. Mouser
June 7th, 1999, Winter Quarter
|
|
Place in this box the first two initials of your last name (e.g., if your last
name is SMITH then you would place "SM" in the box).
DO NOT START UNTIL INSTRUCTED TO DO SO!
|
Question |
Points Possible | Score | Grader's Signature |
| 1. | 15 points | ||
| 2. | 15 points | ||
| 3. | 10 points | ||
| 4. | 10 points | ||
| 5. | 10 points | ||
| 6. | 10 points | ||
| 7. | 30 points | ||
| TOTAL: | 100 points |
1. (15 points) In the first multistep synthesis (formation of tetraphenylnaphthalene), what volume of carbon monoxide (mw = 28.0) is given off if 1.0 gram of benzil (mw = 210) and 1.0 gram of dibenzyl ketone (mw = 210) were used.
Given: mol. wght. of tetraphenylcyclopentadienone = 384 g/mole
Assume: all other reagents are in excess
reaction goes 100% to completion
standard room temperature and pressure
Show your work for full credit.
2. (15 points) Give the detailed mechanism for the formation of benzyne starting from anthranilic acid and isopentyl nitrite.
3. (10 points) Benzyne has 8 pi electrons yet it is still aromatic. Explain.
4. (10 points) What products are formed when thionyl chloride is reacted with water.
5. (10 points) What products are formed when acetyl chloride is reacted with water.
6. (10 points) What is the exact mass of methyl m-nitrobenzoate?
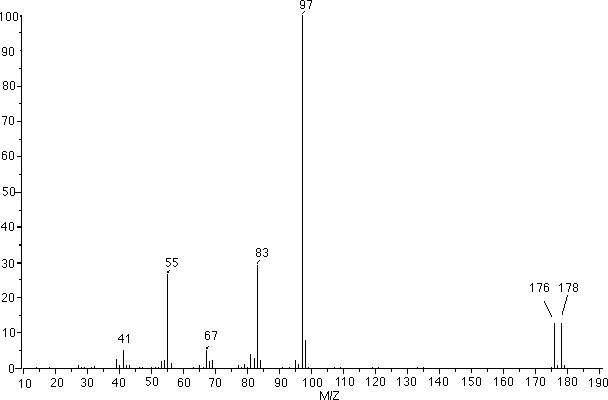
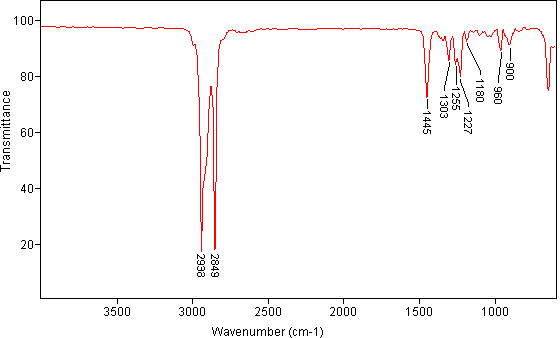
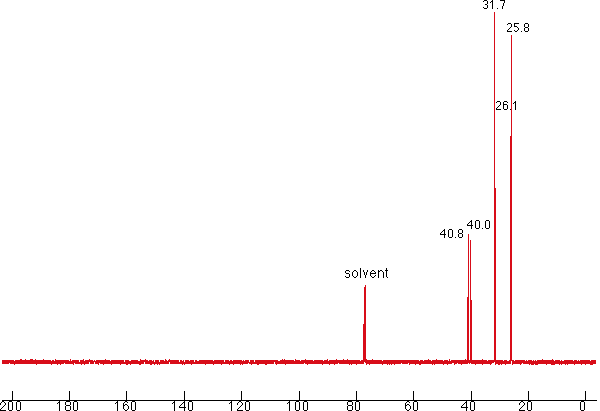
7. Compound W is an unknown. It was determined that the base peak has a molecular formula of C7H13. Given the IR, MS and carbon-13 spectrum, answer the following questions:
a. (3 pts) What is the molecular weight of compound W? _______________
b. (3 pts) What is the molecular formula of compound W _______________
c. (3 pts) Which of the below peaks in the MS is the parent peak? _______________
d.(12 pts) Propose a structure for compound W?
|
|
e. (9pts) Supply structures for in the MS peaks at 83, 97, 179.
|
Peak at 83 |
Peak at 97 |
Peak at 179 |

Mass Spetrum
IR Spectrum of Compound W
Carbon-13 NMR (proton decoupled) of Compound W
Detection Presence of Isotopes:
| Element | Isotope | Exact Mass | Abundance |
| Hydrogen | 1H
2H 3H |
1.00783
2.01410 3.01605 |
99.985
0.015 trace |
| Carbon | 12C
13C 14C |
12.00000
13.00336 14.00324 |
98.90
1.10 trace |
| Nitrogen | 14N
15N |
14.0031
15.0001 |
99.632
0.368 |
| Oxygen | 16O
17O 18O |
15.9949
16.9991 17.9992 |
99.762
0.038 0.200 |
| Fluorine | 19F | 18.9984 | 100.000 |
| Sulfur | 32S
33S 34S |
31.9721
32.9715 33.9679 |
94.93
0.76 4.29 |
| Chlorine | 35Cl
37Cl |
34.9689
36.9659 |
75.77
24.23 |
| Bromine | 79Br
81Br |
78.9183
80.9163 |
50.69
49.31 |
| Iodine | 127I | 126.9045 | 100 |