a.
![]()
b.
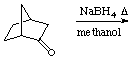
c.
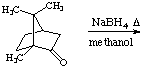
FINAL EXAM, 130AL - KEY
MOUSER, 6/7/99
1. (10 points) At standard atomoshperic pressure, the boiling point of Eugenol is 255°C. What is the boiling point of Eugenol at 60 mm Hg?
2. (10 points) Which is more polar: benzil or dibenzyl ketone? Explain your answer for credit.
3. (10 points) Which is more polar: dibenzyl ketone or tetraphenylcyclopentadienone? Explain your answer for credit.
4. (15 points) 1000 mg of caffeine dissolves in 46 mL of water or 5.5 mL of chloroform. 200 mg of caffeine is added to 10.0 mL of water. This solution is extracted two times with a 5 mL volume of chloroform. SHOW YOUR WORK FOR CREDIT
a. What is the distribution coefficient for caffeine in a water/chloroform solvent system?
b. How much caffeine will be left in the aqueous phase?
c. How much caffeine will be in the combined organic phases (i.e., chloroform phase)?
5.?(15 points) What is the expected major product(s) for the following reactions:
a.
![]()
b.
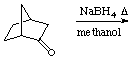
c.
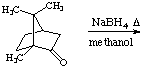
6.(10 points) As a drying agent, anhydrous MgSO4 has a higher "completeness" when compared to anhydrous Na2SO4. Explain what a higher completeness means.
7.(20 points) Complete the below detailed mechanism for the aldol experiment carried out in lab (show all arrow pushing).
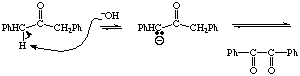 COMPLETE THIS MECHANISM
COMPLETE THIS MECHANISM
8.(20 points) Referring to the below structures answer the following questions.
a. Which of the below compounds are soluble in water:
b. Which of the below compounds are soluble in 5% NaHCO3:
c. Which of the below compounds are soluble in 5% HCl:
d. Which of the below compounds are soluble in 5% NaOH:
9. (20 points) Referring to gas chromatography answer the following questions:
a) define "retention time"
b) how would the retention time of borneol be affected if a polar column was used instead of a nonpolar column?
c) how would the retention time of borneol be affected if the flow rate of the carrier gas was decreased?
d) how would the retention time of borneol be affected if the column temperature was increased?
10.(20 points) How many signals would be expected for the following compounds in the 13C{1H} NMR spectrum?
|
|
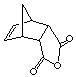 |
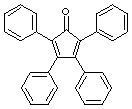 |
|
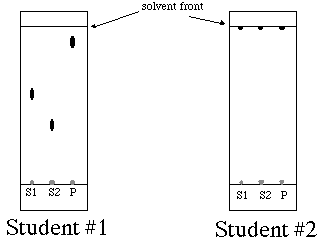
11. (15 points) Both student #1 and student #2 spot their TLC plates with the same components. As the eluting solvent student #1 uses a mixture of toluene and hexane, 4:1, respectively. As the eluting solvent student #2 uses pure toluene. After running the TLC plates (see below), student #1 got good separation of all his spots. However, student #2 noticed that all her spots ended up in the solvent front. Explain
12. (15 points) Student #1 obtained IR spectrum #1 after isolating eugenol from cloves. Student #2 obtained IR spectrum #2 after isolating eugenol from cloves. After comparing their IRs, both student #1 and student #2 noticed there were some subtle differences. How might these differences between the IR's be explained?
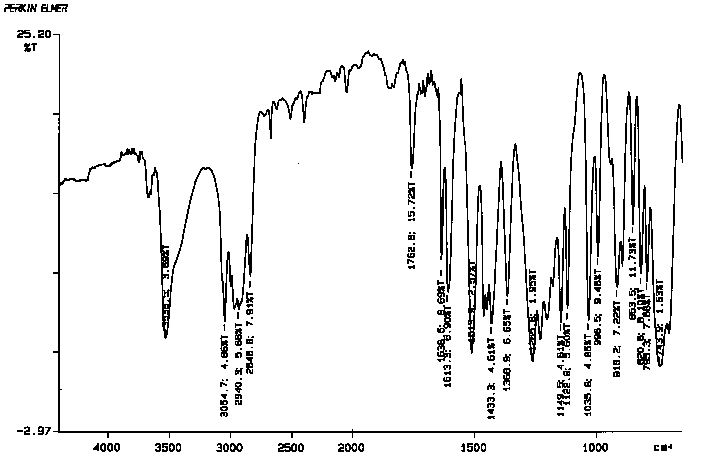 IR Spectrum 1
IR Spectrum 1
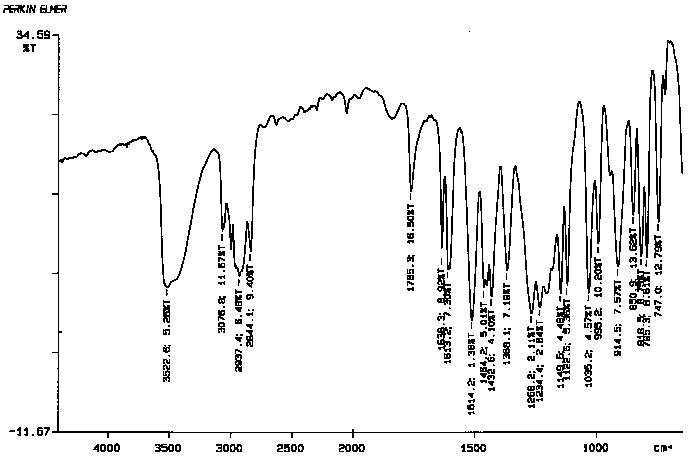 IR Spectrum 2
IR Spectrum 2
13.
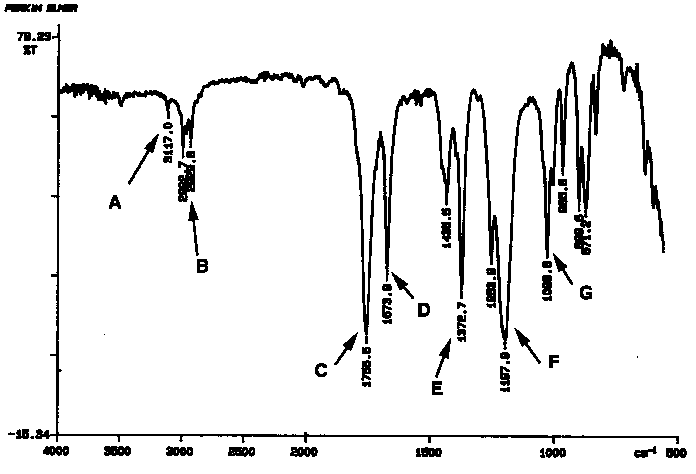
a. (6 points) Which of the following compounds best fits the below IR spectrum (CIRCLE)
b. (14 points) In the below IR spectrum identify peaks A through G. (also mention if it's a stretch or bend).

14. (20 points) Compound Z has a molecular formula C7H13Br. Given the IR, 13C{1H} and DEPT NMR spectrum, propose a structure for compound Z in the below provided box. [No credit will be given for structures placed outside the box.]
|
PLACE YOUR PROPOSED STURTURE HERE! |
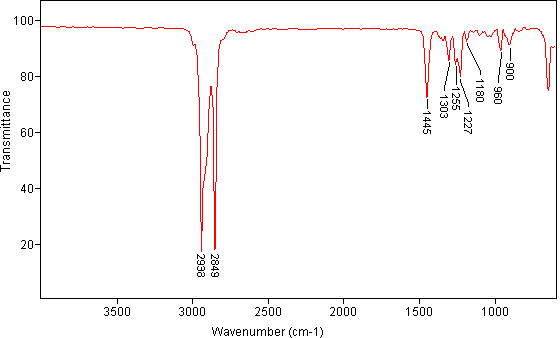
| 13C{1H} NMR
of Compound Z |

|
DEPT TABLE:
# of attached hydrogens
0
1
2
3
DEPT 135
0
up
down
up
DEPT 90
0
up
0
0
DEPT 45
0
up
up
up