last updated on Thu, Apr 26, 2001
30L, Aspirin Experiment
Part 1
1. Only salicylic acid and acetic anhydride should be considered (based on the values given in procedure in the manual). Sulfuric acid is a catalyst and thus should not be considered.
a. Write the balanced equation for the reaction.
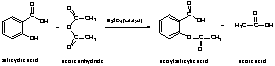
b. Calculate the actual moles used experimentally for that reaction.
moles acetic anhydride = [(5.0 mL) x (1.082 g/mL)] ÷ 102.1 g/mL = 5.3 x10-2 moles
moles salicylic acid = 3.0 g ÷ 138.1 g/mole = 2.2 x10-2 moles
c. The limiting reagent is the compound which is less than the stoichiometric amount required.
Since the stoichiometry for salicylic acid and acetic anhydride is 1:1, acetic anhydride is in excess and salicylic acid is the limiting reagent.
2. 2.2 x10-2 x 180.2 g/mol = 3.9 grams of acetylsalicylic acid
3. Use excess of one of the starting reagents. The procedure calls for excess acetic anhydride. According to Le Chatelier Principle, this should drive the reaction to products.
4. The value given in the question for the solubility of aspirin in ethanol is 40g/100 mL at room temperature. However, one should note that the solubility of aspirin will be greater than this value when in hot ethanol.
Answer: volume of ethanol needed = (3.9 g x 100 mL) ÷ 40 g = 9.75 mL at room temperature
thus, the maximum volume of boiling ethanol needed is 9.75 mL.
special note: aspirin's actual solubility in hot ethanol is ~40g/100mL. However, the above answer was based on the information given.
Part 2
1. ( 0.15 g) / (180.2 g/mol) = 8.32x10-4 mol of aspirin
thus volume needeed = (8.32x10-4 mol of aspirin) / (0.500 mol/L) = 1.7x10-3 L or 1.7 mL
note: the 0.5 M solution has only 1 significant figure. Thus the correct answer would be rounded off to 2 mL. However, in my answer I took the liberty of using a 0.500 M solution (since this is the typical accuracy of a 0.5 M standardrized solution of NaOH).
2. Assumption: the only proton source for the titration is supplied by aspirin (and thus there is no starting material or acetic acid present).
(0.76 mL) x (0.500 mol/L) = 0.38 mmol aspirin
(0.38 mmol) x (180.2 g/mol) = 0.068 g aspirin
thus, % purity = [mass of aspirin / mass of the sample] x 100 = [(0.068 g) / (0.15 g)] x 100 = 46% pure
3. Due to the excess salicylic acid or the by product acetic acid. Both of these are proton sources.
