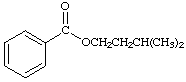
last updatedTuesday, November 19, 2002
Problem set week 9 1. In this week's multistep synthesis, during the Grignard step, water should be excluded as much as possible. Explain (show pertinent chemistry).
2. What role does the dry ice play in the Grignard reaction?
3. Why is it important to place the drying tube on top of the reflux condenser?
4. Why is it important to add the bromobenzene solution slowly?
5. During the Grignard work-up, aqueous sodium hydroxide is used. Why? Show pertinent chemistry.
6. Evaluate the following solvents methanol, acetone, diethylamine, di(n-butyl)ether. Explain briefly why or why they are suitable solvents for theGrignard reaction.
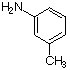
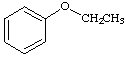
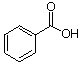
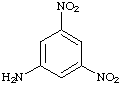
7 a. For each of the below aryl compounds designate (circle) the carbon most likely to undergo electrophilic aromatic substitution.
b. How many 13C{1H} do you expect to observe for each of those compounds?
c. How many signals in the 1H-NMR?
|
|
 |
 |
 |
 |
 |