last updatedThursday, May 15, 2003
Problem set week 8 1. In this week's multistep synthesis, during the Grignard step, water should be excluded as much as possible. a. How do you accomplish that? b. Why is it necessary (show pertinent chemistry)?
2. What role does the dry ice play in the Grignard reaction?
3. What do you have to consider for your setup in regard of the reflux condenser (three points)?
4. How can you minimize the formation of biphenyl in the reaction?
5. During the Grignard work-up, aqueous sodium hydroxide is used. Why? Show pertinent chemistry.
6. Evaluate the following solvents: ethanol, di(iso-propyl)ether, and petroleum ether. Explain briefly why or why not they are suitable solvents for the Grignard reaction that you carry out in the labortaory this week.
7. Using Spartan, show that the ipso-carbon atom in PhBr is electrophilic, while it is nucleophilic in case of PhMgBr. Show appropriate numbers and diagrams.
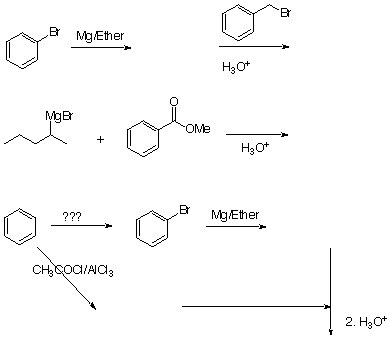
8. Predict the major productsand blanks for the following reactions.
