last updated Thursday, May 9, 2002
Problems Set #7
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. In general, amines react more slowly with anhydrides than acyl halides. Explain.
2. What products are formed when thionyl chloride and acetyl chloride react with water?
3. As the diethylamine solution is added in step 2 of the lab procedure, the reaction mixture becomes white and cloudy. This results from formation of an organic salt. What is this salt? What is the formula of the salt?
4. How could the below amides be differentiated by IR spectroscopy?
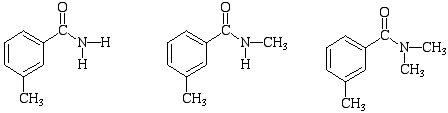
5. Read the following research paper (Jensen, B.L., Fort, R.C., J.Chem.Ed. 2001, 78(4), 538) and summarize the most important findings in 2-3 sentences in your lab notebook.
URL: http://jchemed.chem.wisc.edu/Journal/Issues/2001/Apr/PlusSub/V78N04/p538.pdf
Note: This link only works if you are connect via a UC port e.g. Bruin Online or are located in any computer lab on campus.
6. Suggest a procedure to obtain 1-Methylpyrrolidine from the Me(H)NCH2CH2CH2COOH.
