last updated
Problems Set #4-6
ATTN: answers to the below questions are due at the start of your lab period of Meeting 4; these answers should be part of your pre-lab write-up.
HELPFUL PROCEDURAL HINTS for the GRIGNARD REACTION.
|
Meeting 4 |
Grignard Reaction; do questions 1-5, Start literature work! |
| Meeting 5 | Esterification Reaction/Nitration Reaction; do questions 6-8 |
| Meeting 6 | Finish-up nitration reaction; do questions 9-11 |
| 5-24-02, 5 pm | Draft version of Formal Report due (YH1217) |
| 5-31-02, 5 pm | Final version of Formal Report due (YH1217) |
The draft version is worth 10 points, the final paper 40 points.
1. In this week's multistep synthesis, during the Grignard step, water should be excluded as much as possible. Explain (show pertinent chemistry).
2. What purpose does the dry ice play in the Grignard reaction?
3. Why is it important to place the drying tube on top of the reflux condenser?
4. During the Grignard work-up, aqueous sodium hydroxide is used. Why? Show pertinent chemistry.
5. Which of the following solvents (ethanol, acetone, toluene, di(n-propyl)ether)) would not be a suitable solvent for a Grignard reaction? Explain briefly why.
6. In the esterification step, what is the function of the methanol and the sulfuric acid?
7. Outline a separation scheme for the isolation of the ester. How could you recover any unreacted benzoic acid?
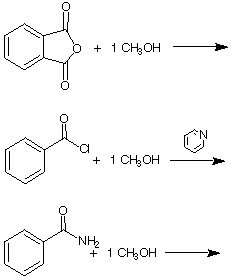
8. Complete the following reactions (show all products!)

![]()
9.
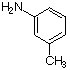
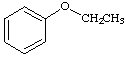
a. For each of the below aryl compounds designate (circle) the carbon most likely to undergo electrophilic aromatic substitution.
b. How many 13C{1H} do you expect to observe for each of those compounds?
c. How many signals in the 1H-NMR?
|
|
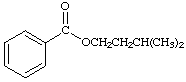 |
 |
 |
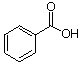 |
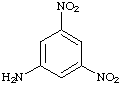 |
10. During the nitration step, explain why does the amount of the dinitro derivative increases with increase in temperature of the reaction mixture? Explain.
11. What is the function of the sulfuric acid in the nitration reaction? Explain.