last updated
ATTN: Answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. During the synthesis of the Jacobsen catalyst, you are asked to pass a slow air stream through your reaction mixture. Why?
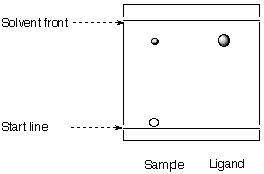
2. A student obtains the following TLC spectrum during the synthesis of the Jacobsen ligand.

a. Calculate the Rf-values. What can be said about the polarity of the compounds?
b. Identify the individual spots.
c. Which conclusion can the student draw about the progress of his reaction?
3. Referring to the structure of the Jacobsen catalyst, how can you describe the coordination figure around the Mn-atom (hint: Look up the X-ray structure in the literature)?
4. Which changes do you expect to observe in the IR spectrum of the Mn-complex (in comparison with the ligand)?
5. Even though the ligand has two hydroxyl groups, the IR spectrum does not show any strong peaks in the range between 3200-3600 cm-1. Rationalize this observation.
6. How can you account for the yellow color of the Jacobsen ligand even though both starting materials are white?
7. Write a balanced equation for the oxidation of the alcohol with Ce(OH)3(OOH). Why is it important that the starting materials are dry?
8. During the work-up of the catalyst, heptane is used. Why?