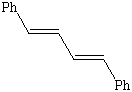
Problems Set #3
ATTN: answers to the below questions are due at the start of your lab period; these answers should be part of your pre-lab write-up.
1. Supply the structure of cinnamaldehyde (show the stereochemistry).
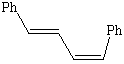
2. Name the following compounds.
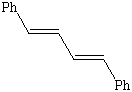

![]()
3. In this week's experiment which of the above compounds are not an expected product? Explain.
4. Why do we use absolute ethanol instead of 95% ethanol in the intial part of the experiment?
5. What combination of carbonyl compound and ylide are needed to prepare the following compounds
| a. | 1,5-heptadiene |
| b. | Methyl styrene |
| c. | 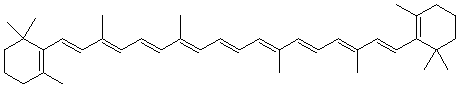 beta-Carotene beta-Carotene
use the below dialdehyde as the carbonyl source |
6. What are the major advantages of the Wittig reaction in order to form alkenes?
7. Read the following article (Gillois, J.; Guillerm, G.; Stephen, E.; Vo-Quang, L., ( J. Chem. Educ. 1980, 57 , l6l)). How does the proposed synthesis strategy differ from the one use in the lab? Where do you see advantages or disadvantages for the procedure described in the reference?