last updated
ATTN: answers to the below questions are due at the start of your second meeting; these answers should be part of your pre-lab write-up. There will also be a quiz in meeting 2.
1. Referring to step 2 (page 34), answer the following questions:
a. Determine the molarity of the formaldehyde solution (36%wt) used in this reaction.
b. Why does the sodium hydroxide has to be dissolved completely before the formaldehyde solution is added? Show pertinent equations.
c. How does TLC help you to evaluate the outcome of your reaction? Explain briefly.
d. Why is a solvent mixture used for the TLC and not only hexane?
2. Referring to step 3a (page 35), answer the following questions:
a. How do you explain the color change during the reaction?
b. Why is it important to add the 30% hydrogen peroxide solution slowly?
c. How many grams of the product do you expect for this reaction assuming a 90% yield? Show calculations.
d. A student obtained a total yield of 120% for the reaction. Which conclusion can the student draw from this observation? Can he continue with step 3b?
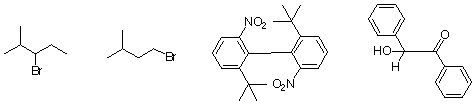
3. Which of the following molecules is chiral? If so, why?

4. Find two examples (other than the ones mentioned in the reader) in the literature where enantiomers have different properties e.g. odor, pharmaceutical activity. Provide references with your examples.