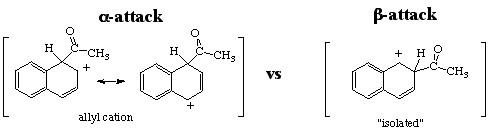

you need to first understand the expected product for acylation of naphthalene.
Naphthalene preferably undergoes electrophilic aromatic substitution at the alpha-position
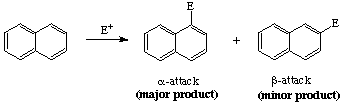
Question: Why is the alpha-attack favored over the beta-attack for naphthalene? Look at the arenium ion formed for both attacks and determine which is most stable.